Pfizer and BioNTech have applied to the FDA for authorization of their COVID-19 booster shot for children ages 5-11, according to an update from Pfizer.
Maldonado advises the American Academy of Pediatrics and has also helped to test the vaccine for Pfizer.
At the same time, experts have argued that the primary vaccines are still protecting most people, including children, from severe illness. Offit is also an adviser to the FDA. He said that without evidence that a third dose boosts protection against serious illness, “I don’t see a clear reason to give a third dose now.”, which could potentially include reviews by their independent advisory panels. In January, the FDA authorized Pfizer booster doses for children 12-15 without calling a meeting of its outside expert panel.
Indonesia Berita Terbaru, Indonesia Berita utama
Similar News:Anda juga dapat membaca berita serupa dengan ini yang kami kumpulkan dari sumber berita lain.
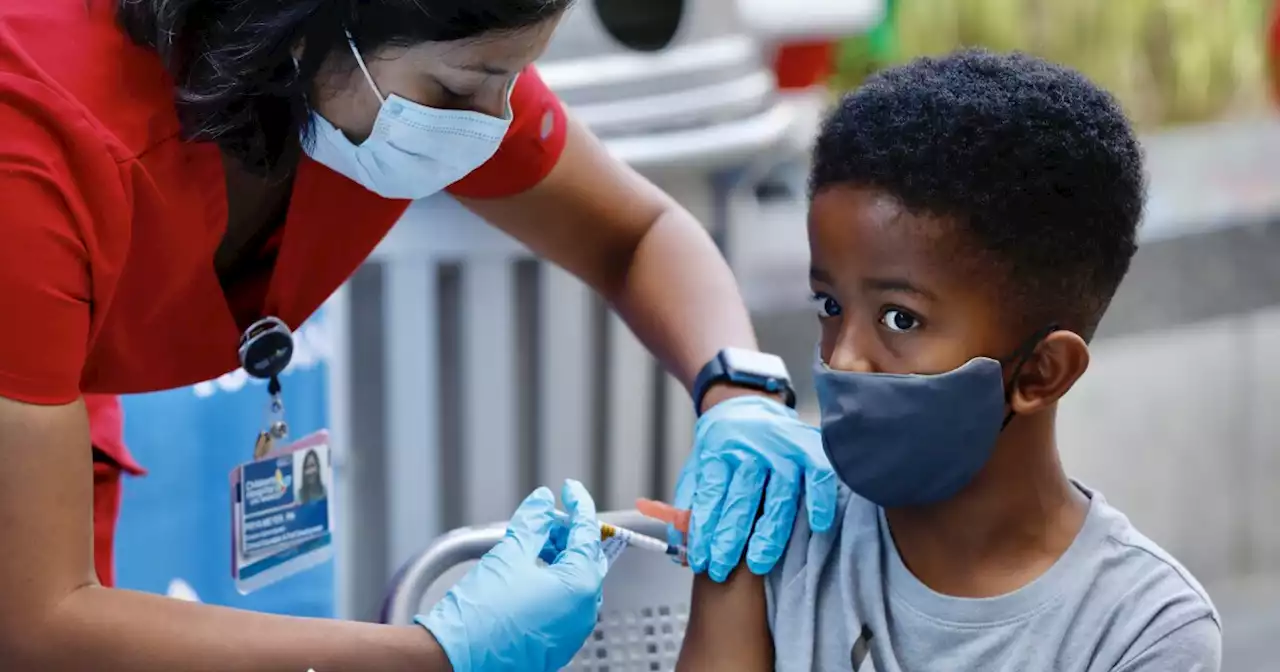 Pfizer asks FDA to clear COVID-19 booster shot for children ages 5 to 11Pfizer asked U.S. regulators for emergency-use authorization of a booster shot of its COVID-19 vaccine in children ages 5 to 11.
Pfizer asks FDA to clear COVID-19 booster shot for children ages 5 to 11Pfizer asked U.S. regulators for emergency-use authorization of a booster shot of its COVID-19 vaccine in children ages 5 to 11.
Baca lebih lajut »
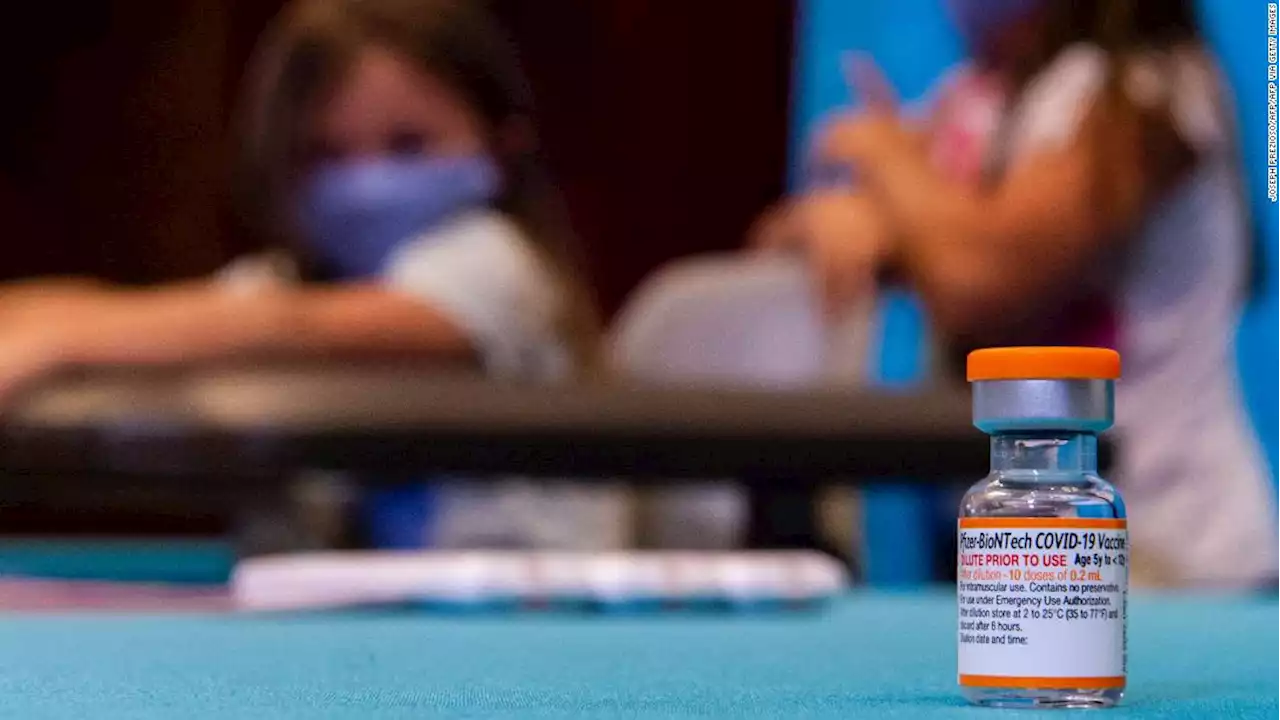 Pfizer requests FDA authorization for Covid-19 booster for kids 5 through 11Pfizer and BioNTech said Tuesday that they have submitted an application to the US Food and Drug Administration for emergency use authorization of a booster dose of Covid-19 vaccine for children 5 through 11.
Pfizer requests FDA authorization for Covid-19 booster for kids 5 through 11Pfizer and BioNTech said Tuesday that they have submitted an application to the US Food and Drug Administration for emergency use authorization of a booster dose of Covid-19 vaccine for children 5 through 11.
Baca lebih lajut »
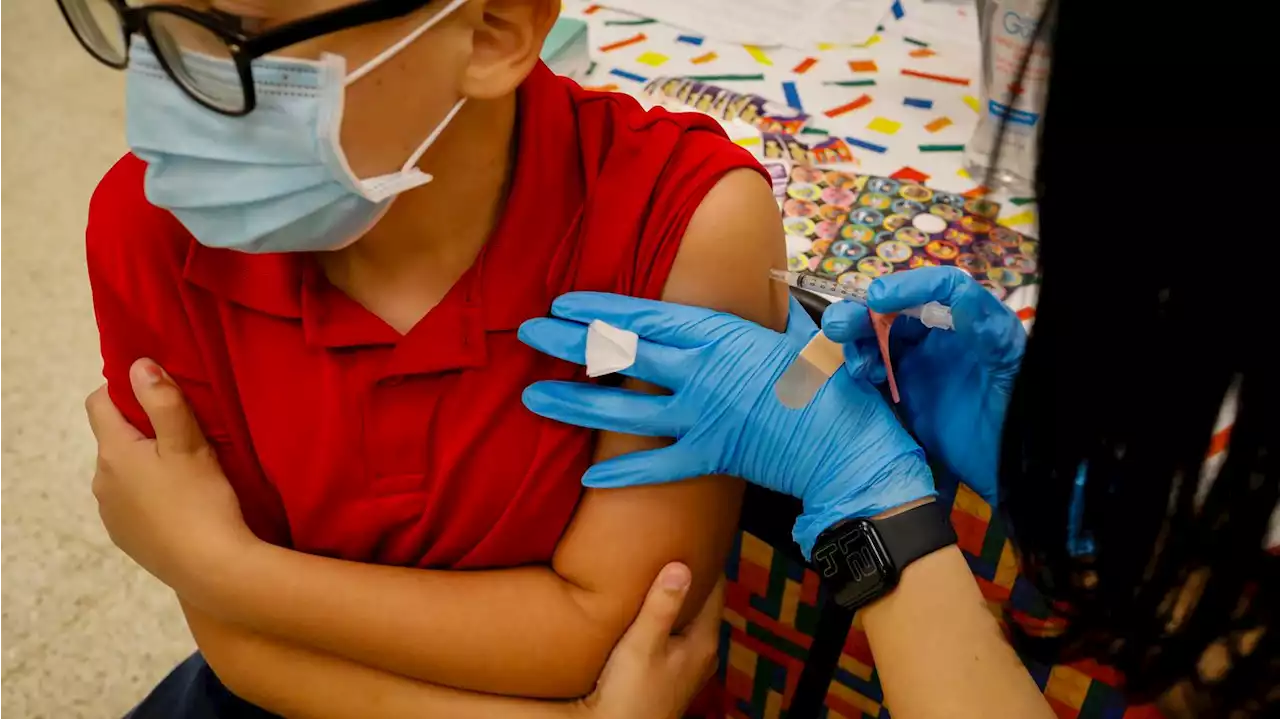 Pfizer asks FDA to authorize COVID booster for children 5 to 11If approved, it would be the first booster accessible to children of this age group, who were hit hard by Omicron.
Pfizer asks FDA to authorize COVID booster for children 5 to 11If approved, it would be the first booster accessible to children of this age group, who were hit hard by Omicron.
Baca lebih lajut »
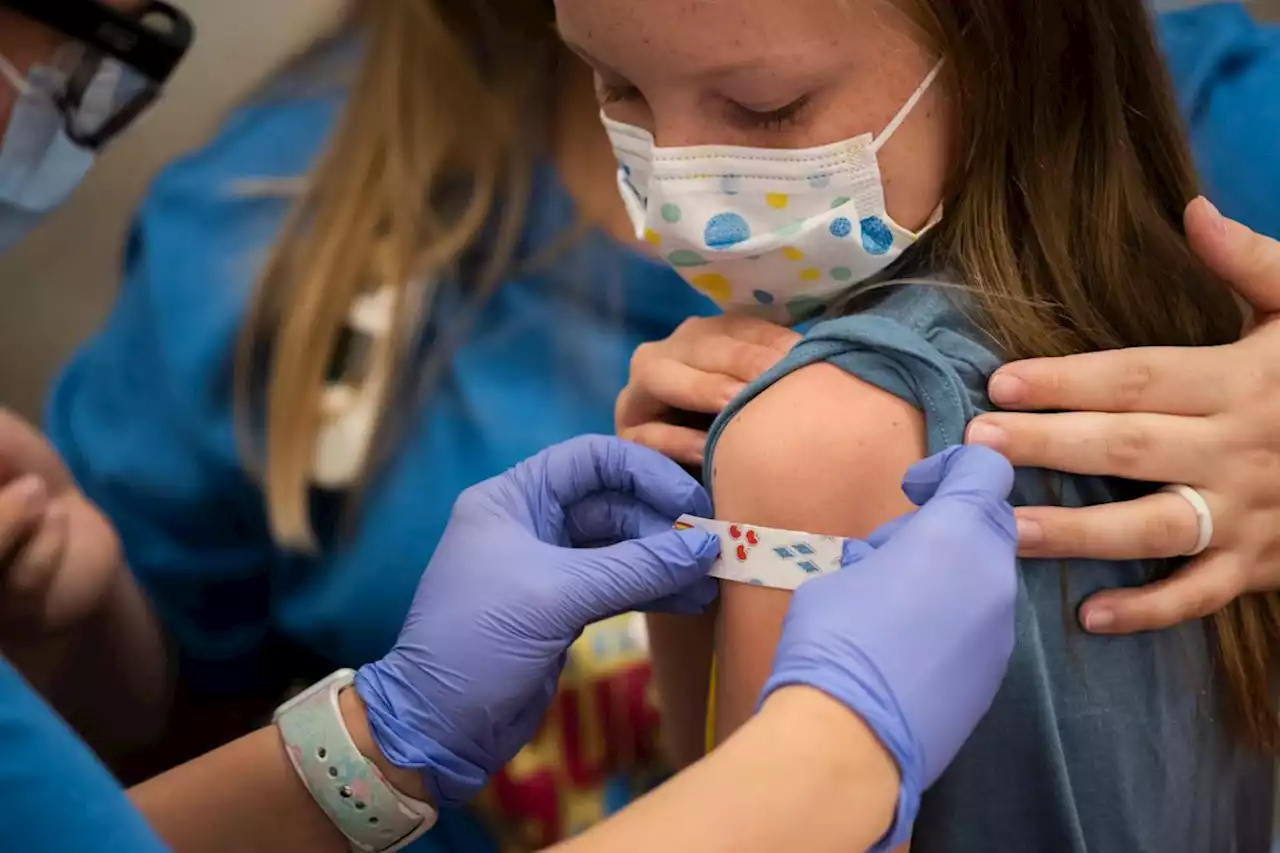 Pfizer-BioNTech seeks FDA authorization for a COVID booster shot for kids 5-11Pfizer and BioNTech have requested that the Food and Drug Administration authorize a COVID-19 booster shot for children ages 5-11.
Pfizer-BioNTech seeks FDA authorization for a COVID booster shot for kids 5-11Pfizer and BioNTech have requested that the Food and Drug Administration authorize a COVID-19 booster shot for children ages 5-11.
Baca lebih lajut »
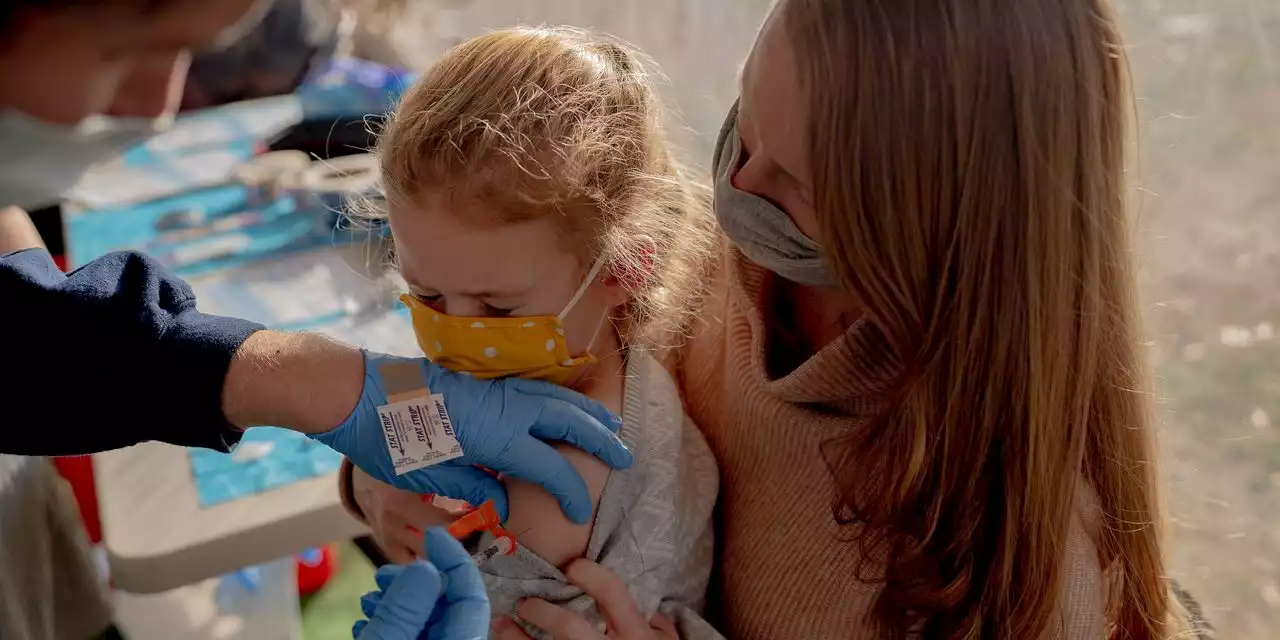 Pfizer Asks FDA to Authorize Covid-19 Booster for 5- to 11-Year-OldsPfizer Inc. and partner BioNTech SE said earlier this month that a third shot safely generated a strong immune response in the youngsters.
Pfizer Asks FDA to Authorize Covid-19 Booster for 5- to 11-Year-OldsPfizer Inc. and partner BioNTech SE said earlier this month that a third shot safely generated a strong immune response in the youngsters.
Baca lebih lajut »
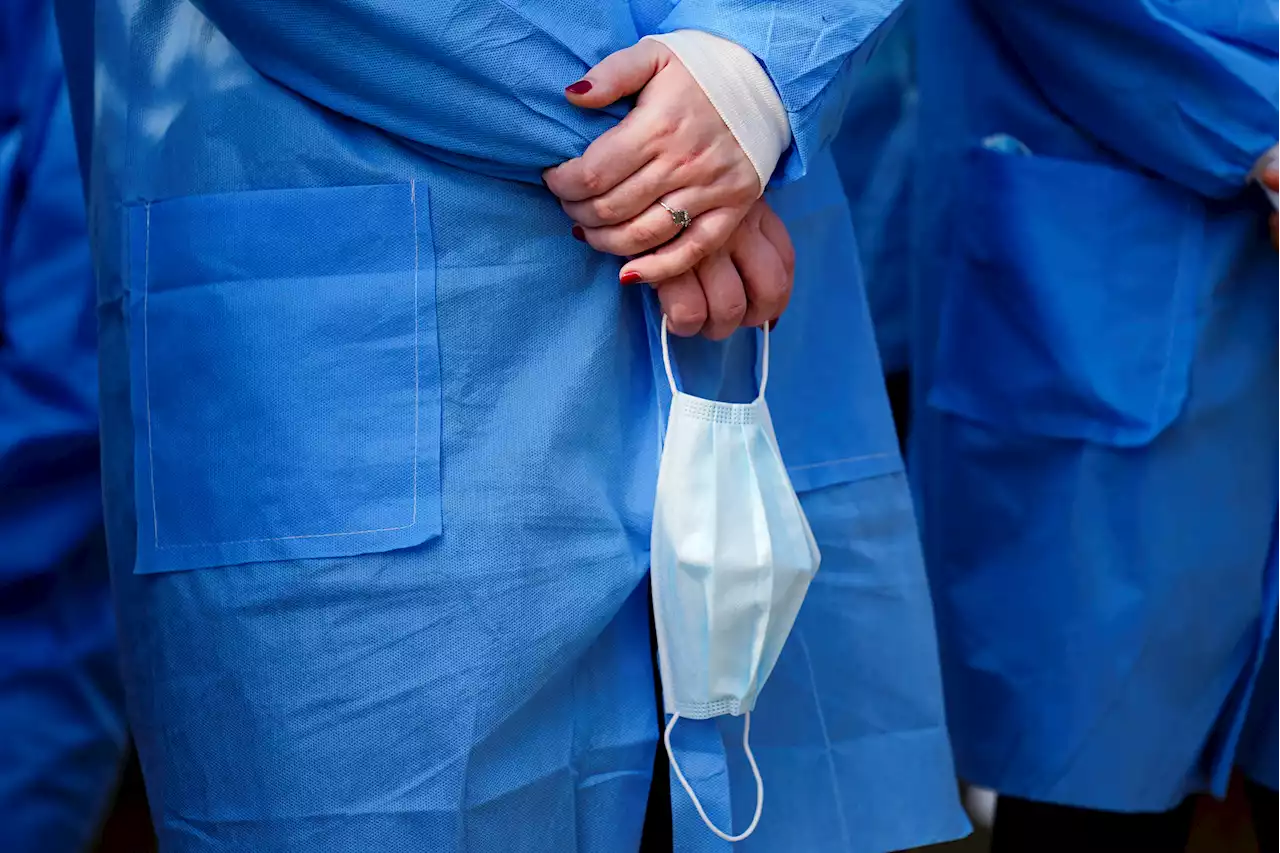 Pfizer Asks FDA to Authorize Third Dose for Children 5 to 11 Years OldPfizer on Tuesday asked the Food and Drug Administration to authorize a third dose of its COVID vaccine for children ages 5- to 11-years-old. The application comes after Pfizer released data earlier this month from a small lab study of blood samples from 30 kids in the age group, which showed a 36-fold increase in antibody levels against the omicron variant…
Pfizer Asks FDA to Authorize Third Dose for Children 5 to 11 Years OldPfizer on Tuesday asked the Food and Drug Administration to authorize a third dose of its COVID vaccine for children ages 5- to 11-years-old. The application comes after Pfizer released data earlier this month from a small lab study of blood samples from 30 kids in the age group, which showed a 36-fold increase in antibody levels against the omicron variant…
Baca lebih lajut »
