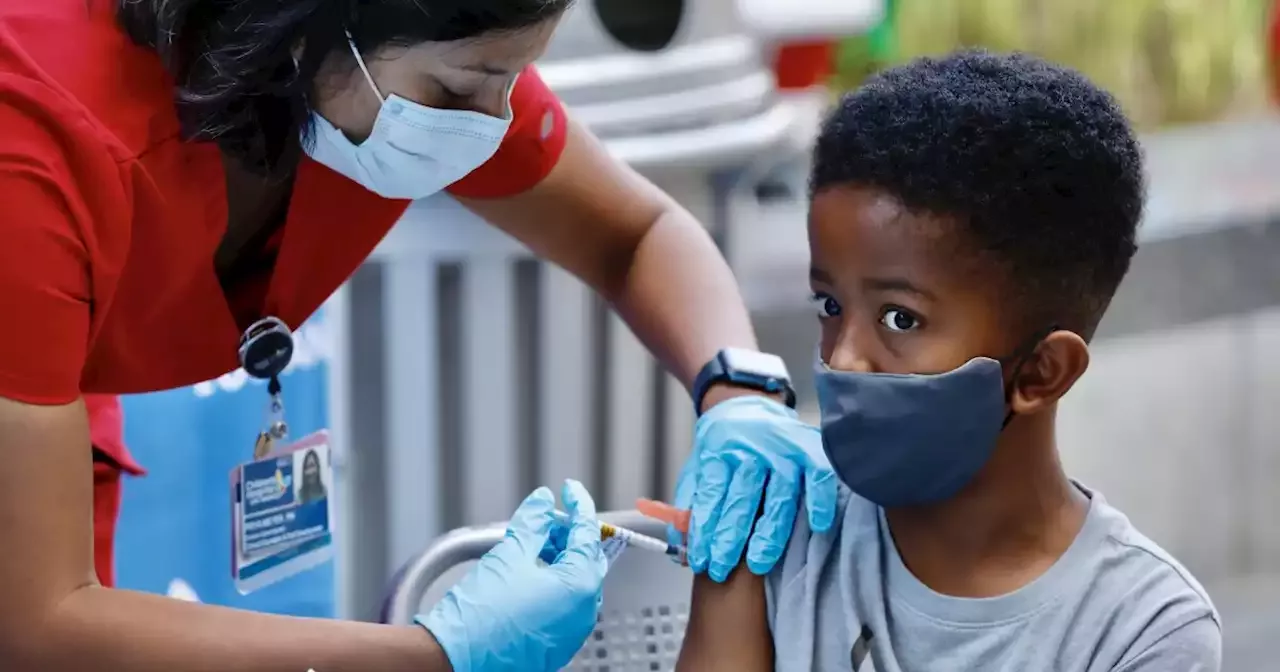
Pfizer asked U.S. regulators for emergency-use authorization of a booster shot of its COVID-19 vaccine in children ages 5 to 11.
Pfizer Inc. has asked U.S. regulators for emergency-use authorization of a booster shot of its COVID-19 vaccine in children ages 5 to 11, setting in motion an effort to provide extra protection to kids.
Pfizer and partner BioNTech submitted data to the Food and Drug Administration from a late-stage study that showed a third-dose booster shot, given about 6 months after the second dose, provided a strong immune response. The companies also plan to submit data to the European Medicines Agency and other regulators around the world, according to a statement Tuesday. No new safety concerns were identified, they said.
The U.S. campaign to immunize children has tapered off, with 28% of children ages 5 to 11 fully vaccinated, according to the Centers for Disease Control and Prevention. Fewer than a quarter of adolescents 12 to 17, who are already eligible for boosters, have received one. That bodes poorly for a booster campaign among the youngest children, experts say
Indonesia Berita Terbaru, Indonesia Berita utama
Similar News:Anda juga dapat membaca berita serupa dengan ini yang kami kumpulkan dari sumber berita lain.
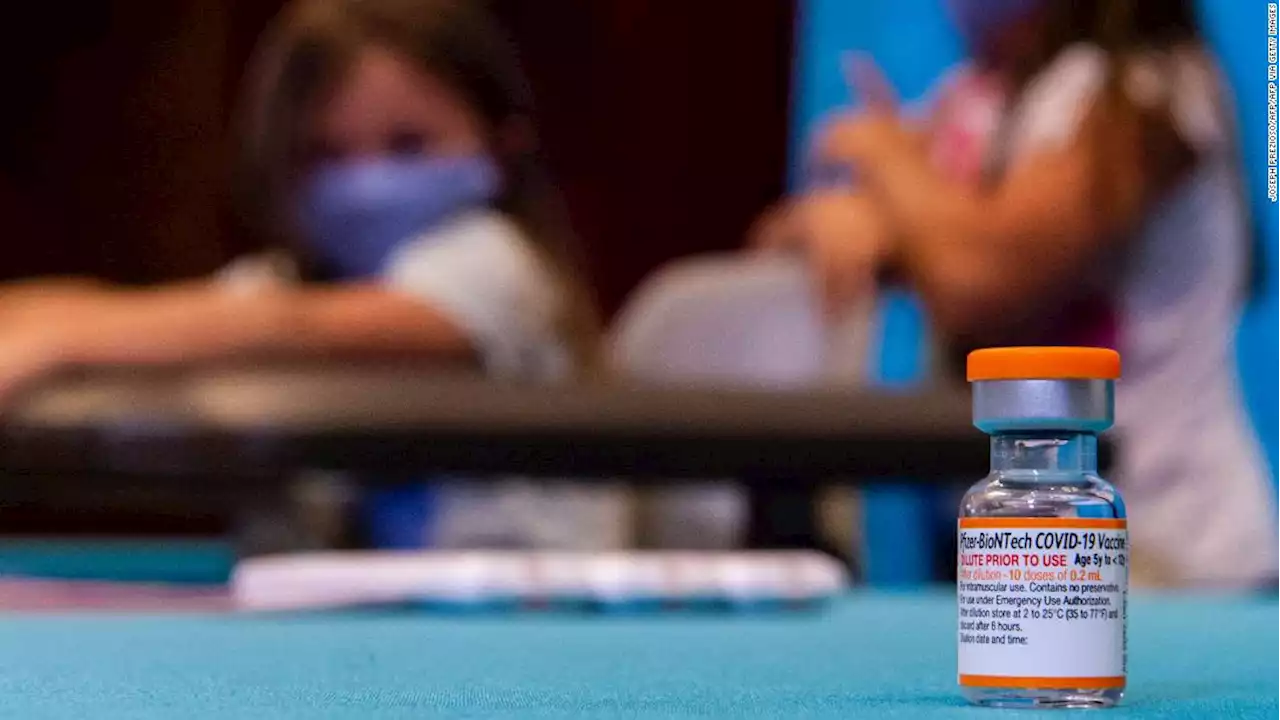 Pfizer requests FDA authorization for Covid-19 booster for kids 5 through 11Pfizer and BioNTech said Tuesday that they have submitted an application to the US Food and Drug Administration for emergency use authorization of a booster dose of Covid-19 vaccine for children 5 through 11.
Pfizer requests FDA authorization for Covid-19 booster for kids 5 through 11Pfizer and BioNTech said Tuesday that they have submitted an application to the US Food and Drug Administration for emergency use authorization of a booster dose of Covid-19 vaccine for children 5 through 11.
Baca lebih lajut »
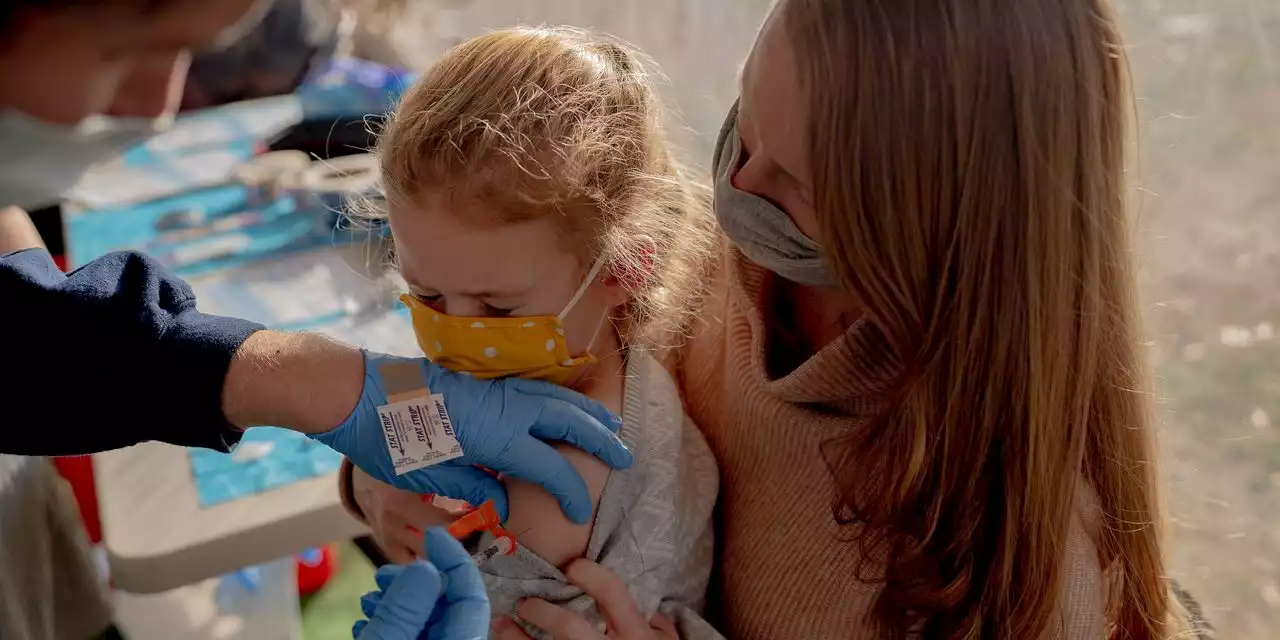 Pfizer Asks FDA to Authorize Covid-19 Booster for 5- to 11-Year-OldsPfizer Inc. and partner BioNTech SE said earlier this month that a third shot safely generated a strong immune response in the youngsters.
Pfizer Asks FDA to Authorize Covid-19 Booster for 5- to 11-Year-OldsPfizer Inc. and partner BioNTech SE said earlier this month that a third shot safely generated a strong immune response in the youngsters.
Baca lebih lajut »
 Biden announces efforts to improve access to Pfizer COVID-19 treatmentThe Biden administration said it will attempt to save more lives with Pfizer’s effective treatment for COVID-19 by nearly doubling the number of pharmacies that stock the pills and setting up federally supported “test-to-treat” sites that offer the drug to people who test positive.
Biden announces efforts to improve access to Pfizer COVID-19 treatmentThe Biden administration said it will attempt to save more lives with Pfizer’s effective treatment for COVID-19 by nearly doubling the number of pharmacies that stock the pills and setting up federally supported “test-to-treat” sites that offer the drug to people who test positive.
Baca lebih lajut »
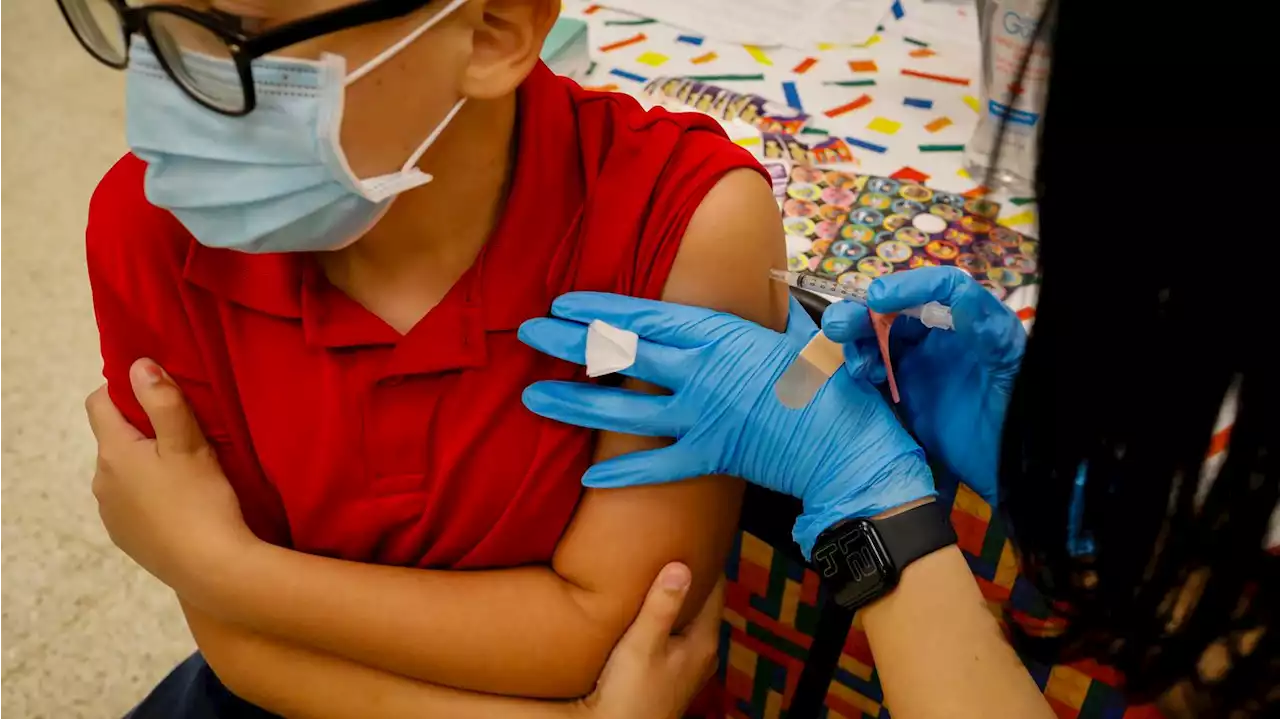 Pfizer asks FDA to authorize COVID booster for children 5 to 11If approved, it would be the first booster accessible to children of this age group, who were hit hard by Omicron.
Pfizer asks FDA to authorize COVID booster for children 5 to 11If approved, it would be the first booster accessible to children of this age group, who were hit hard by Omicron.
Baca lebih lajut »
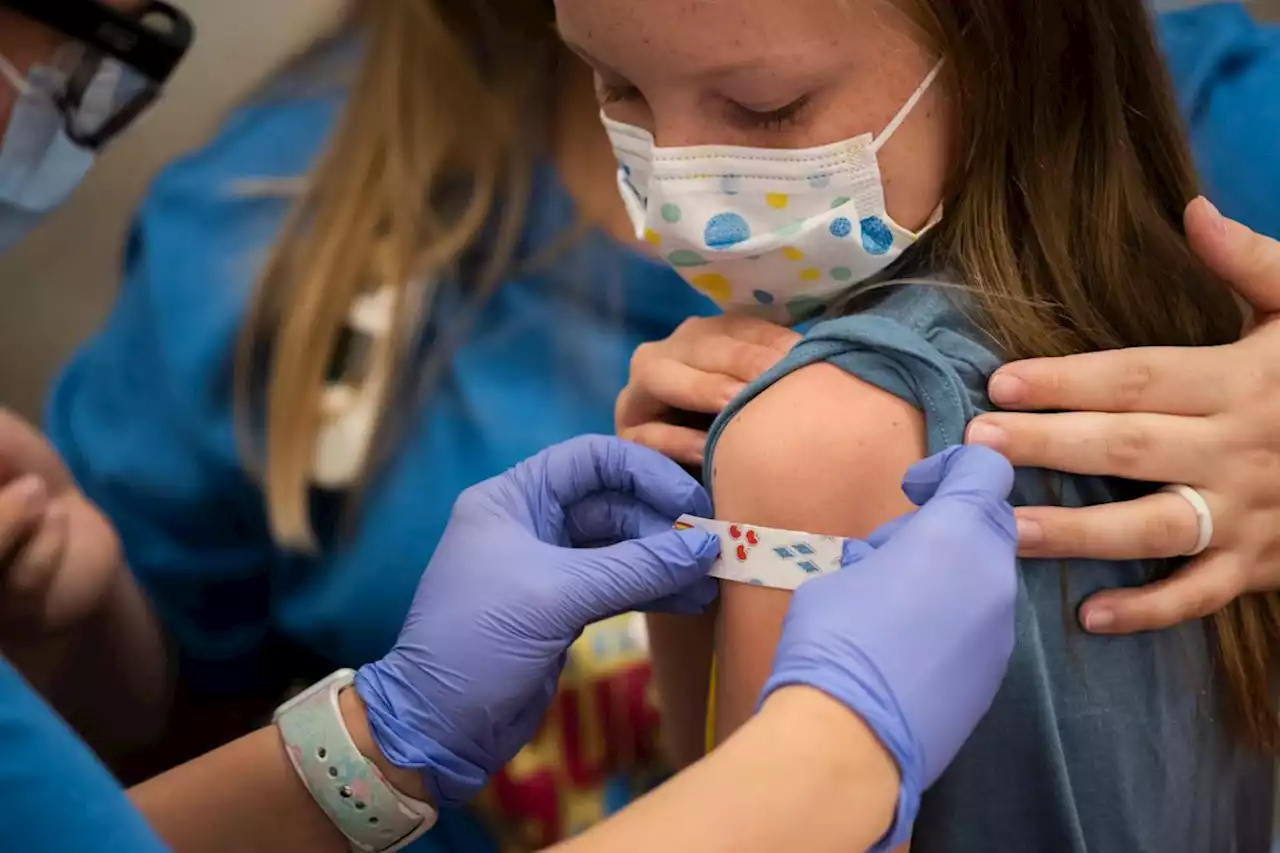 Pfizer-BioNTech seeks FDA authorization for a COVID booster shot for kids 5-11Pfizer and BioNTech have requested that the Food and Drug Administration authorize a COVID-19 booster shot for children ages 5-11.
Pfizer-BioNTech seeks FDA authorization for a COVID booster shot for kids 5-11Pfizer and BioNTech have requested that the Food and Drug Administration authorize a COVID-19 booster shot for children ages 5-11.
Baca lebih lajut »
 FDA approves COVID drug for children, China deals with uphill COVID battle, and more COVID newsThe US Food and Drug Administration announced Monday that it has expanded approval of the COVID-19 drug remdesivir to treat patients as young as 28 days old. Here's that and more COVID news.
FDA approves COVID drug for children, China deals with uphill COVID battle, and more COVID newsThe US Food and Drug Administration announced Monday that it has expanded approval of the COVID-19 drug remdesivir to treat patients as young as 28 days old. Here's that and more COVID news.
Baca lebih lajut »