Moderna on Thursday is expected to formally ask the FDA to authorize its Covid-19 vaccine for children under the age of 6
Moderna on Thursday is expected to formally ask the Food and Drug Administration to authorize its Covid-19 vaccine for children under the age of 6, three people with knowledge of the matter told POLITICO.
“Just remember that we can’t actually finish our reviews until we actually have complete applications in the FDA,” Marks testified to the Senate HELP Committee. “We will proceed with all due speed, once we have complete applications, some of these are complicated because they are relatively covering larger swaths of the pediatric population than others,” he added.
“Many families of young kids are hesitant to resume their normal activities until the vaccine is available to their kids,” Colorado Gov. Jared Polis said in an interview. “Just put the facts in front of parents and let them make that decision.” The White House, for its part, has made clear that the decision is solely up to the FDA, the people said. Some officials over the past week have even gone out of their way not to discuss the vaccines with FDA regulators, for fear of being accused of political interference.
Indonesia Berita Terbaru, Indonesia Berita utama
Similar News:Anda juga dapat membaca berita serupa dengan ini yang kami kumpulkan dari sumber berita lain.
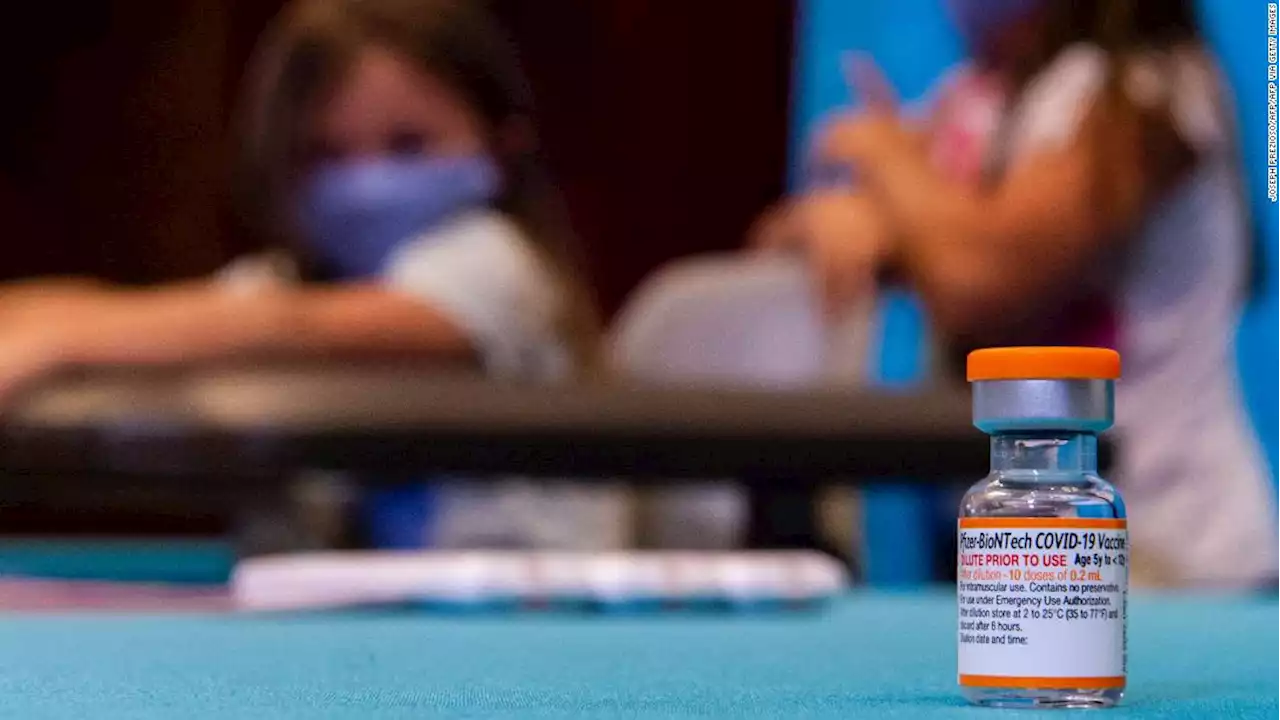 Pfizer requests FDA authorization for Covid-19 booster for kids 5 through 11Pfizer and BioNTech said Tuesday that they have submitted an application to the US Food and Drug Administration for emergency use authorization of a booster dose of Covid-19 vaccine for children 5 through 11.
Pfizer requests FDA authorization for Covid-19 booster for kids 5 through 11Pfizer and BioNTech said Tuesday that they have submitted an application to the US Food and Drug Administration for emergency use authorization of a booster dose of Covid-19 vaccine for children 5 through 11.
Baca lebih lajut »
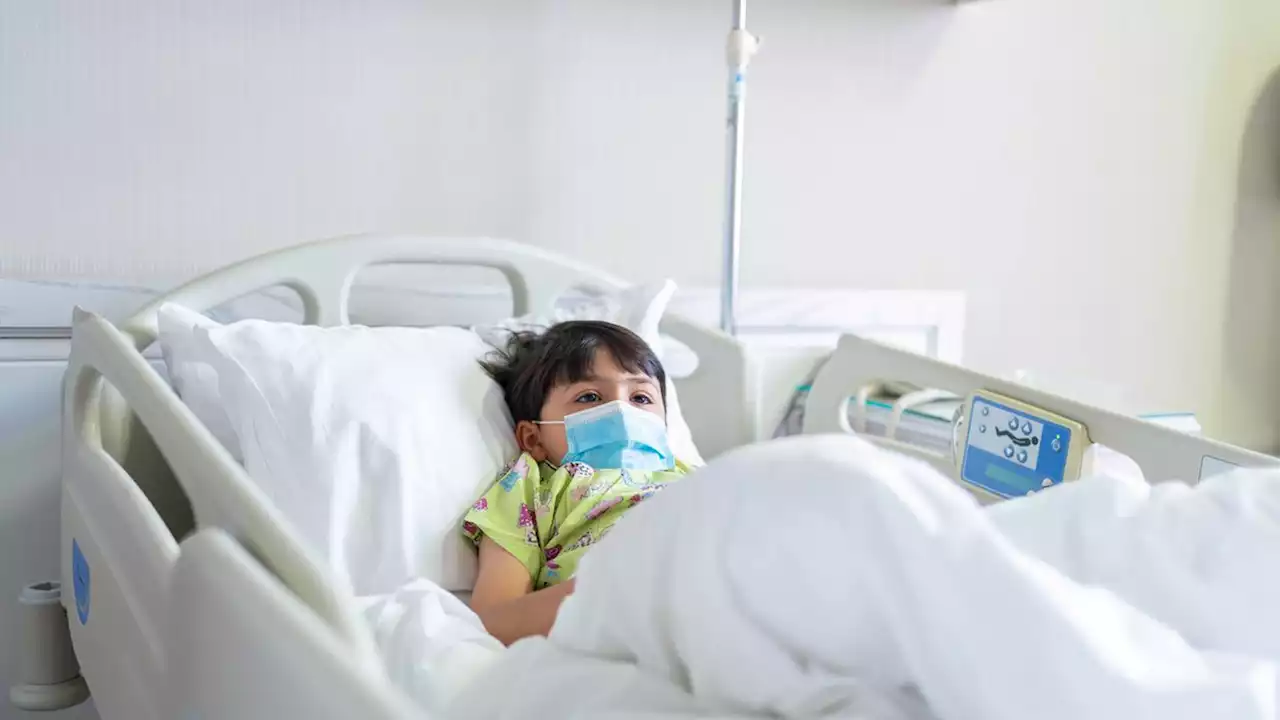 Coronavirus: FDA approves first COVID-19 treatment for children younger than 12The FDA said Monday it expanded the approval of Veklury to include pediatric patients in certain circumstances.
Coronavirus: FDA approves first COVID-19 treatment for children younger than 12The FDA said Monday it expanded the approval of Veklury to include pediatric patients in certain circumstances.
Baca lebih lajut »
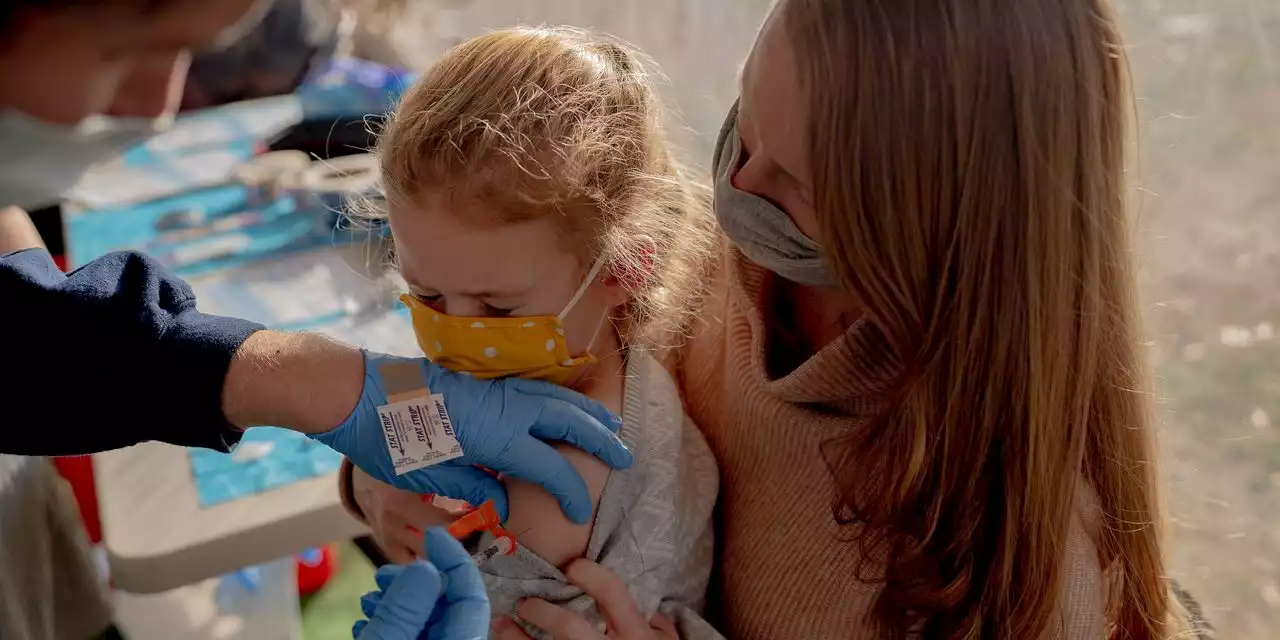 Pfizer Asks FDA to Authorize Covid-19 Booster for 5- to 11-Year-OldsPfizer Inc. and partner BioNTech SE said earlier this month that a third shot safely generated a strong immune response in the youngsters.
Pfizer Asks FDA to Authorize Covid-19 Booster for 5- to 11-Year-OldsPfizer Inc. and partner BioNTech SE said earlier this month that a third shot safely generated a strong immune response in the youngsters.
Baca lebih lajut »
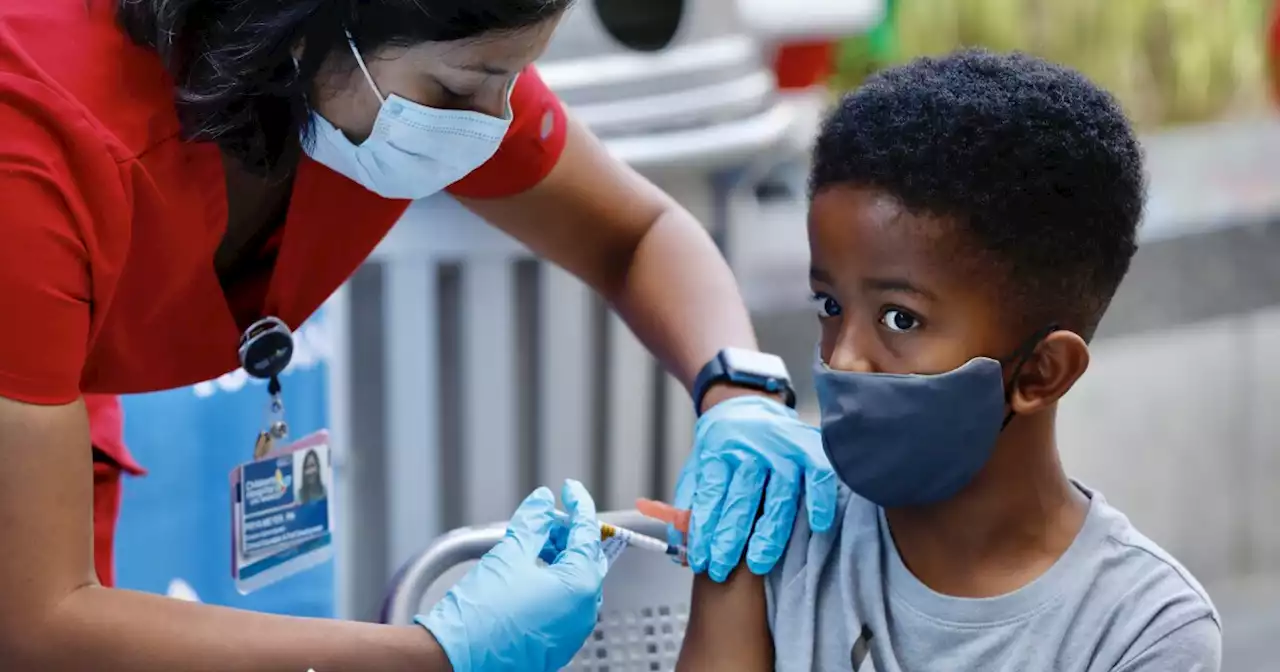 Pfizer asks FDA to clear COVID-19 booster shot for children ages 5 to 11Pfizer asked U.S. regulators for emergency-use authorization of a booster shot of its COVID-19 vaccine in children ages 5 to 11.
Pfizer asks FDA to clear COVID-19 booster shot for children ages 5 to 11Pfizer asked U.S. regulators for emergency-use authorization of a booster shot of its COVID-19 vaccine in children ages 5 to 11.
Baca lebih lajut »
 FDA approves Remdesivir to treat COVID in young childrenThe antiviral drug is given as an injection. It was previously approved for patients 12 years of age and older.
FDA approves Remdesivir to treat COVID in young childrenThe antiviral drug is given as an injection. It was previously approved for patients 12 years of age and older.
Baca lebih lajut »
 FDA Approves First COVID Treatment for Use in KidsThe FDA has approved the antiviral remdesivir as the first COVID-19 treatment for young children.
FDA Approves First COVID Treatment for Use in KidsThe FDA has approved the antiviral remdesivir as the first COVID-19 treatment for young children.
Baca lebih lajut »
