FDA advisers on Thursday backed the use of Sanofi and partner AstraZeneca's experimental antibody to prevent respiratory syncytial virus infections in infants.
- The US Food and Drug Administration advisers on Thursday backed the use of Sanofi and partner AstraZeneca's experimental antibody to prevent respiratory syncytial virus infections in infants.
Swedish Orphan Biovitrum's treatment Synagis is currently the only approved preventive therapy in the United States for high-risk infants against RSV, a leading cause of hospitalizations during an infant's first year of life. Two studies showed the therapy was effective in preventing lower respiratory tract infections, the FDA staff reviewers had said on Tuesday.
Indonesia Berita Terbaru, Indonesia Berita utama
Similar News:Anda juga dapat membaca berita serupa dengan ini yang kami kumpulkan dari sumber berita lain.
 US FDA panel backs Sanofi-AstraZeneca's preventive RSV therapyThe U.S. Food and Drug Administration advisers on Thursday backed the use of Sanofi and partner AstraZeneca's experimental antibody to prevent respiratory syncytial virus (RSV) infections in infants.
US FDA panel backs Sanofi-AstraZeneca's preventive RSV therapyThe U.S. Food and Drug Administration advisers on Thursday backed the use of Sanofi and partner AstraZeneca's experimental antibody to prevent respiratory syncytial virus (RSV) infections in infants.
Baca lebih lajut »
 FDA advisors recommend AstraZeneca antibody to protect babies from RSVIf the Food and Drug Administration approves nirsevimab, it will be the first medical intervention available in the U.S. that can protect infants from RSV.
FDA advisors recommend AstraZeneca antibody to protect babies from RSVIf the Food and Drug Administration approves nirsevimab, it will be the first medical intervention available in the U.S. that can protect infants from RSV.
Baca lebih lajut »
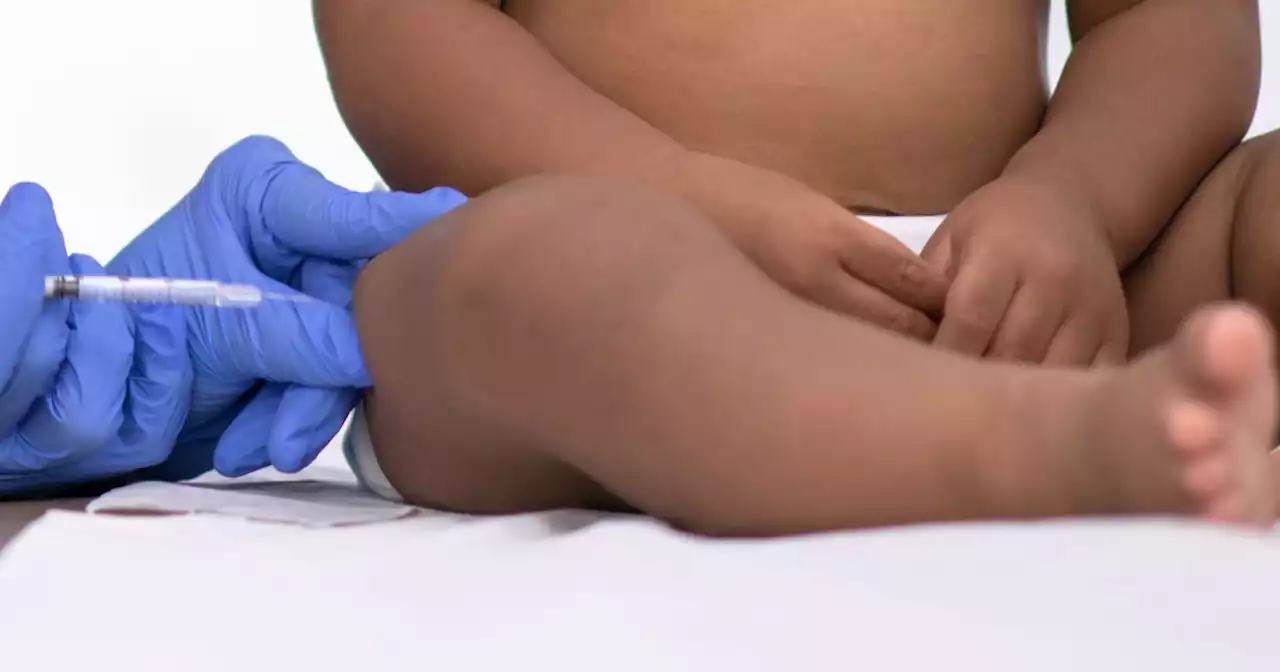 FDA panel recommends injectable drug to prevent RSV in infantsThe monoclonal antibody shot would be given to babies during or before their first RSV season, as well as to high-risk children up to age 2 entering their second RSV season.
FDA panel recommends injectable drug to prevent RSV in infantsThe monoclonal antibody shot would be given to babies during or before their first RSV season, as well as to high-risk children up to age 2 entering their second RSV season.
Baca lebih lajut »
 FDA panel endorses antibody therapy to protect against RSV in infantsIt is designed to be given in a single shot at birth or a baby’s first season of RSV.
FDA panel endorses antibody therapy to protect against RSV in infantsIt is designed to be given in a single shot at birth or a baby’s first season of RSV.
Baca lebih lajut »
 5 things to know for June 9: Trump, Air quality, Stocks, RSV, Ukraine | CNNHere are 5⃣ things to know today. 1⃣ Trump indicted 2⃣ Air quality set to improve 3⃣ S&P 500 in bull market territory 4⃣ RSV treatment for infants 5⃣ Ukraine dam collapse
5 things to know for June 9: Trump, Air quality, Stocks, RSV, Ukraine | CNNHere are 5⃣ things to know today. 1⃣ Trump indicted 2⃣ Air quality set to improve 3⃣ S&P 500 in bull market territory 4⃣ RSV treatment for infants 5⃣ Ukraine dam collapse
Baca lebih lajut »
