The U.S. Food and Drug Administration advisers on Thursday backed the use of Sanofi and partner AstraZeneca's experimental antibody to prevent respiratory syncytial virus (RSV) infections in infants.
The advisers voted unanimously in favor of using the antibody, nirsevimab, in newborns and infants to prevent infections in their first RSV season.
In a separate 19-2 vote, the panel backed the therapy's use in children aged up to two years who are vulnerable to severe illness through their second RSV season.treatment Synagis is currently the only approved preventive therapy in the United States for high-risk infants against RSV, a leading cause of hospitalizations during an infant's first year of life.
Unlike Synagis, which is given as monthly injections, nirsevimab is a long-acting therapy expected to be given once every season to prevent infection regardless of additional medical conditions in infants. "This is probably the closest thing to an RSV vaccine that we have and it really moves the field forward," said panel member Nimish Patel.
Two studies showed the therapy was effective in preventing lower respiratory tract infections, the FDA staff reviewers had
Indonesia Berita Terbaru, Indonesia Berita utama
Similar News:Anda juga dapat membaca berita serupa dengan ini yang kami kumpulkan dari sumber berita lain.
 FDA advisors recommend AstraZeneca antibody to protect babies from RSVIf the Food and Drug Administration approves nirsevimab, it will be the first medical intervention available in the U.S. that can protect infants from RSV.
FDA advisors recommend AstraZeneca antibody to protect babies from RSVIf the Food and Drug Administration approves nirsevimab, it will be the first medical intervention available in the U.S. that can protect infants from RSV.
Baca lebih lajut »
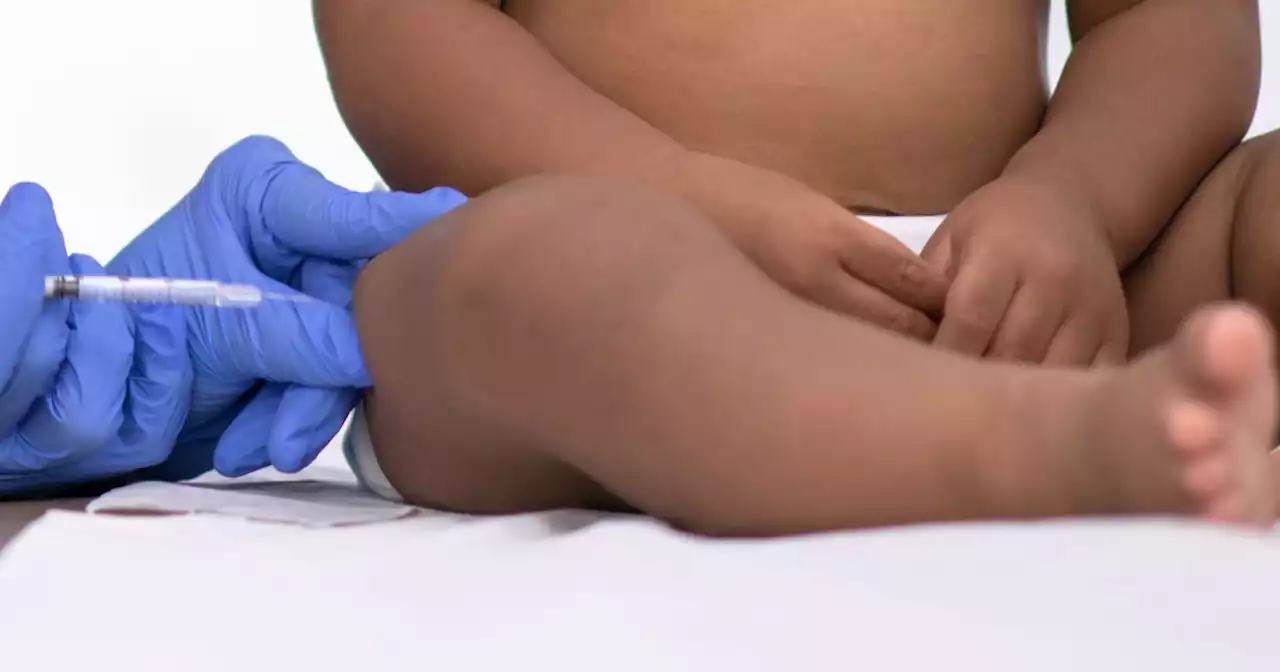 FDA panel recommends injectable drug to prevent RSV in infantsThe monoclonal antibody shot would be given to babies during or before their first RSV season, as well as to high-risk children up to age 2 entering their second RSV season.
FDA panel recommends injectable drug to prevent RSV in infantsThe monoclonal antibody shot would be given to babies during or before their first RSV season, as well as to high-risk children up to age 2 entering their second RSV season.
Baca lebih lajut »
 FDA OKs Letermovir to Prevent CMV in Kidney TransplantsLetermovir was shown to be just as effective as valganciclovir in preventing CMV in kidney transplant recipients in new study published in JAMA; results prompt additional FDA approval for this indication.
FDA OKs Letermovir to Prevent CMV in Kidney TransplantsLetermovir was shown to be just as effective as valganciclovir in preventing CMV in kidney transplant recipients in new study published in JAMA; results prompt additional FDA approval for this indication.
Baca lebih lajut »
 FDA Finalizes Limit on How Much Arsenic Can Be in Apple JuiceFederal regulators have finalized new guidance on how much inorganic arsenic can be present in apple juice, in an effort to limit the exposure of infants and young children to this environmental contaminant.
FDA Finalizes Limit on How Much Arsenic Can Be in Apple JuiceFederal regulators have finalized new guidance on how much inorganic arsenic can be present in apple juice, in an effort to limit the exposure of infants and young children to this environmental contaminant.
Baca lebih lajut »
 FDA clears Lynparza for prostate cancer treatmentThe Food and Drug Administration cleared a new cancer-fighting drug called Lynparza for prostate cancer treatment.
FDA clears Lynparza for prostate cancer treatmentThe Food and Drug Administration cleared a new cancer-fighting drug called Lynparza for prostate cancer treatment.
Baca lebih lajut »
 FDA warns against self-diagnosis, bogus treatments for this common skin conditionThe Food and Drug Administration is warning against self-diagnosis and self-treatment for a common skin condition - a viral infection often called 'water warts.'
FDA warns against self-diagnosis, bogus treatments for this common skin conditionThe Food and Drug Administration is warning against self-diagnosis and self-treatment for a common skin condition - a viral infection often called 'water warts.'
Baca lebih lajut »
