FDA advisors move COVID-19 shots closer for kids under 5
“This is a long-awaited vaccine,” said one panel member, Dr. Jay Portnoy of Children’s Hospital in Kansas City, Mo. “There are so many parents who are absolutely desperate to get this vaccine and I think we owe it to them to give them a choice to have the vaccine if they want to.”An average of about 15,900 new coronavirus infections a day were reported over the last week across California, health officials say.
, and noted that 442 children under 4 have died during the pandemic. That’s far fewer than adult deaths, but should not be dismissed in considering the need for vaccinating the youngest kids, he said.FDA reviewers said both brands of vaccine appear to be safe and effective for children as young as 6 months old in analyses posted before the all-day meeting. Side effects, including fever and fatigue, were generally minor in both, and less common than seen in adults.
“That is a really important point,”’ said Dr. Jesse Goodman of Georgetown University, a former FDA vaccine chief. “You can’t compare the vaccines directly.” Pfizer’s vaccine is for children 6 months through 4 years; Moderna’s vaccine is for 6 months through 5 years.
Indonesia Berita Terbaru, Indonesia Berita utama
Similar News:Anda juga dapat membaca berita serupa dengan ini yang kami kumpulkan dari sumber berita lain.
 Dogs Can Sniff Out COVID-19 and Signs of Long COVID, Studies SuggestAlmost any dog can be trained to do this type of detection in a matter of weeks, researchers say
Dogs Can Sniff Out COVID-19 and Signs of Long COVID, Studies SuggestAlmost any dog can be trained to do this type of detection in a matter of weeks, researchers say
Baca lebih lajut »
 FDA advisers back Moderna's COVID-19 vaccine for older kidsA government advisory panel has endorsed a second brand of COVID-19 vaccine for school-age children and teens.
FDA advisers back Moderna's COVID-19 vaccine for older kidsA government advisory panel has endorsed a second brand of COVID-19 vaccine for school-age children and teens.
Baca lebih lajut »
 FDA advisers back Moderna's COVID-19 vaccine for kids ages 6 and upThe Food and Drug Administration's outside experts voted unanimously that Moderna's vaccine is safe and effective enough to give kids ages 6 to 17. If the FDA agrees, it would become the second option for those children, joining Pfizer's vaccine.
FDA advisers back Moderna's COVID-19 vaccine for kids ages 6 and upThe Food and Drug Administration's outside experts voted unanimously that Moderna's vaccine is safe and effective enough to give kids ages 6 to 17. If the FDA agrees, it would become the second option for those children, joining Pfizer's vaccine.
Baca lebih lajut »
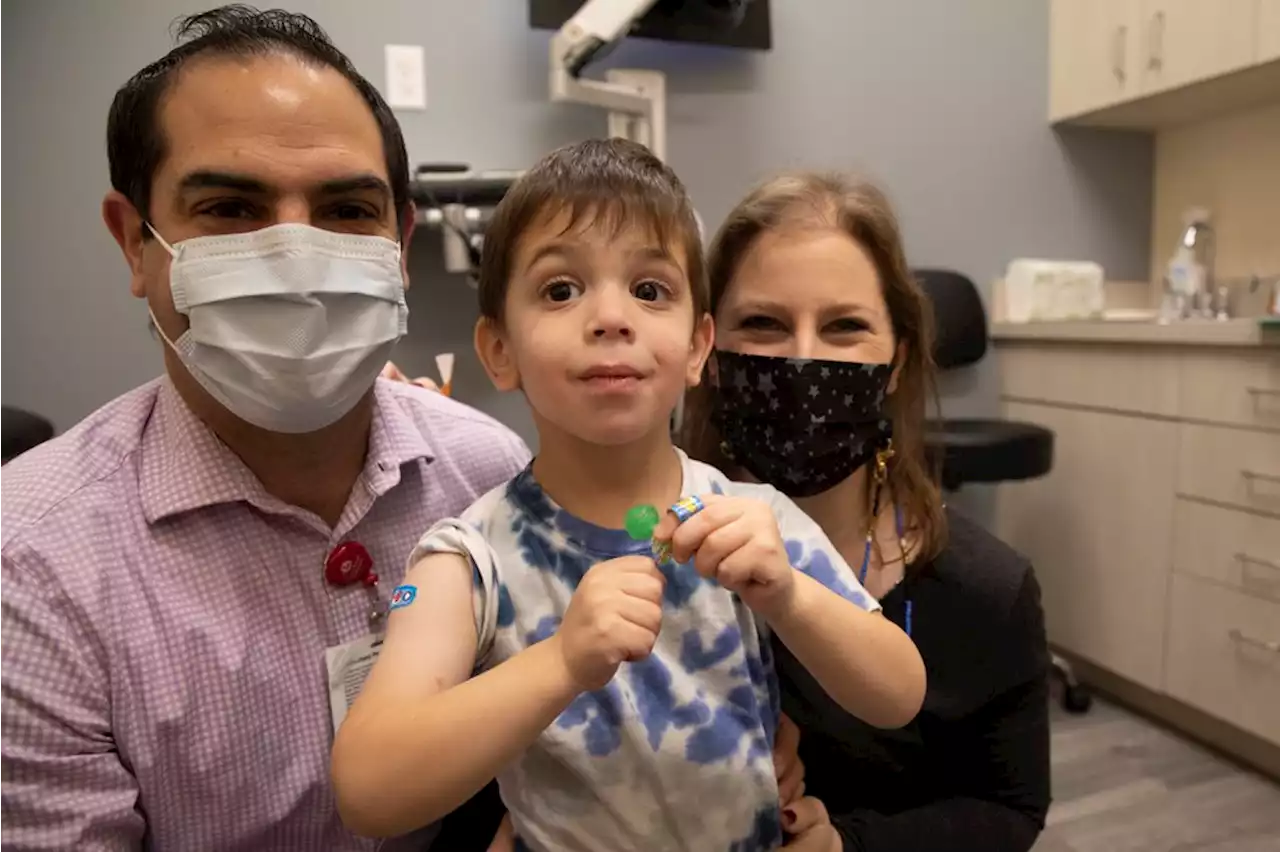 FDA advisers back Moderna’s COVID-19 vaccine for teens, younger kidsA government advisory panel Tuesday endorsed a second brand of COVID-19 vaccine for school-age children and teens.
FDA advisers back Moderna’s COVID-19 vaccine for teens, younger kidsA government advisory panel Tuesday endorsed a second brand of COVID-19 vaccine for school-age children and teens.
Baca lebih lajut »
 FDA advisers back Moderna's COVID-19 vaccine for older kidsA government advisory panel has endorsed a second brand of COVID-19 vaccine for school-age children and teens. The expert panel agreed Tuesday that the vaccine made by Moderna is safe and effective enough to give to U.S. kids ages 6 to 17.
FDA advisers back Moderna's COVID-19 vaccine for older kidsA government advisory panel has endorsed a second brand of COVID-19 vaccine for school-age children and teens. The expert panel agreed Tuesday that the vaccine made by Moderna is safe and effective enough to give to U.S. kids ages 6 to 17.
Baca lebih lajut »
 FDA panel backs Moderna's COVID-19 vaccine for kids ages 6-17The FDA's outside experts voted unanimously that Moderna’s COVID vaccine is safe and effective enough to give kids ages 6 to 17.
FDA panel backs Moderna's COVID-19 vaccine for kids ages 6-17The FDA's outside experts voted unanimously that Moderna’s COVID vaccine is safe and effective enough to give kids ages 6 to 17.
Baca lebih lajut »
