The White House previously said vaccines for young children may start being available next week.
The Food and Drug Administration’s outside experts voted unanimously that Moderna’s vaccine is safe and effective enough to give kids ages 6 to 17. If the FDA agrees, it would become the second option for those children, joining Pfizer’s vaccine.tot-sized shots from Moderna and Pfizer for the littlest kidsModerna’s COVID-19 vaccine has long been available for adults in the U.S. and elsewhere and more than three dozen countries offer it to older children, too.
“I believe that this will provide families an important option” and may be particularly important for families who live in areas where coronavirus spread is increasing, said another panel member, Dr. Ofer Levy of Boston Children’s Hospital. “That clearly needs to be watched closely going forward as we expand the use of the vaccine,” said Dr. Mark Sawyer, a panel member from the University of California, San Diego’s medical school.
Vaccine effectiveness was estimated at 93% for the teens, and 77% for the younger children, according to the FDA analysis. However, the research was done when earlier versions of the coronavirus were causing most U.S. infections, before more contagious versions emerged. It’s also based on a limited number of COVID-19 cases, making the estimates a bit rough.
Indonesia Berita Terbaru, Indonesia Berita utama
Similar News:Anda juga dapat membaca berita serupa dengan ini yang kami kumpulkan dari sumber berita lain.
 Dogs Can Sniff Out COVID-19 and Signs of Long COVID, Studies SuggestAlmost any dog can be trained to do this type of detection in a matter of weeks, researchers say
Dogs Can Sniff Out COVID-19 and Signs of Long COVID, Studies SuggestAlmost any dog can be trained to do this type of detection in a matter of weeks, researchers say
Baca lebih lajut »
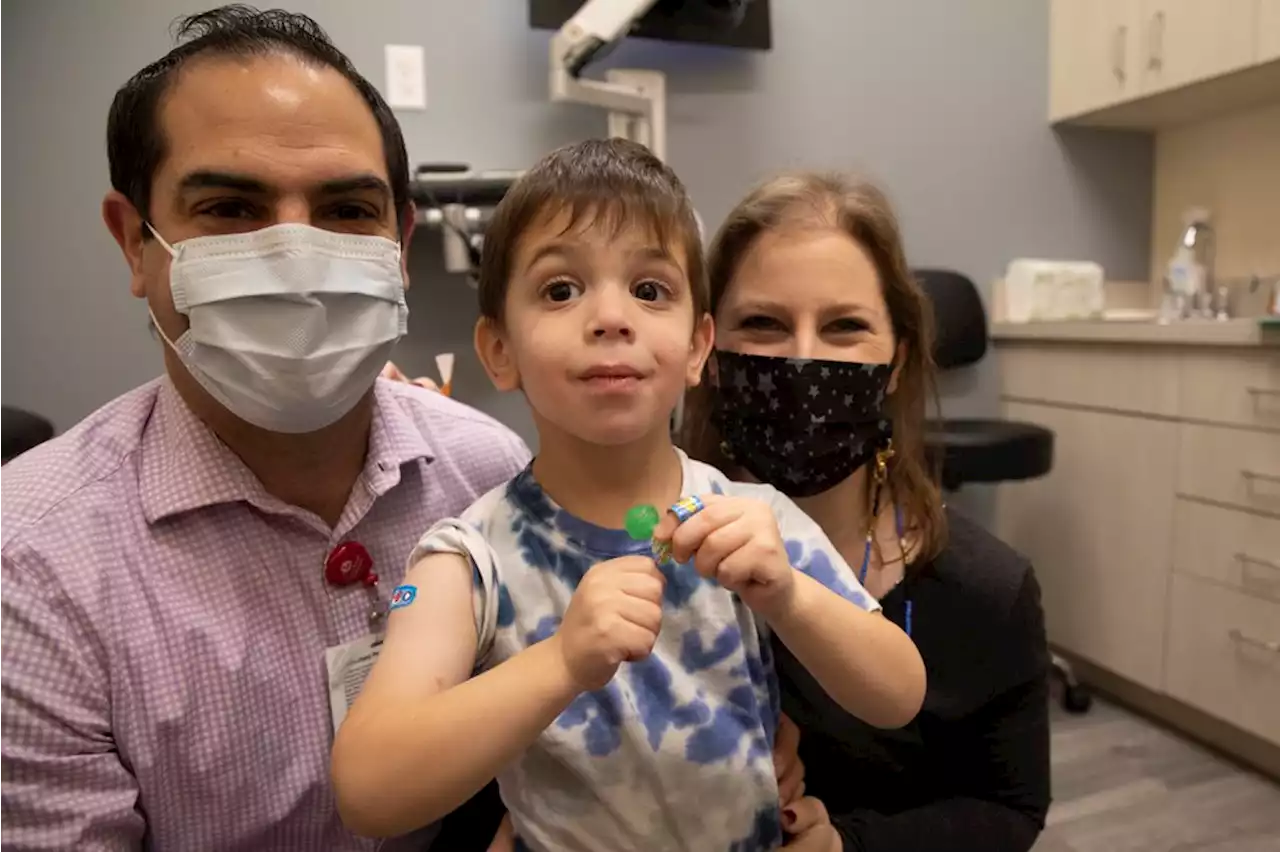 FDA advisers back Moderna’s COVID-19 vaccine for teens, younger kidsA government advisory panel Tuesday endorsed a second brand of COVID-19 vaccine for school-age children and teens.
FDA advisers back Moderna’s COVID-19 vaccine for teens, younger kidsA government advisory panel Tuesday endorsed a second brand of COVID-19 vaccine for school-age children and teens.
Baca lebih lajut »
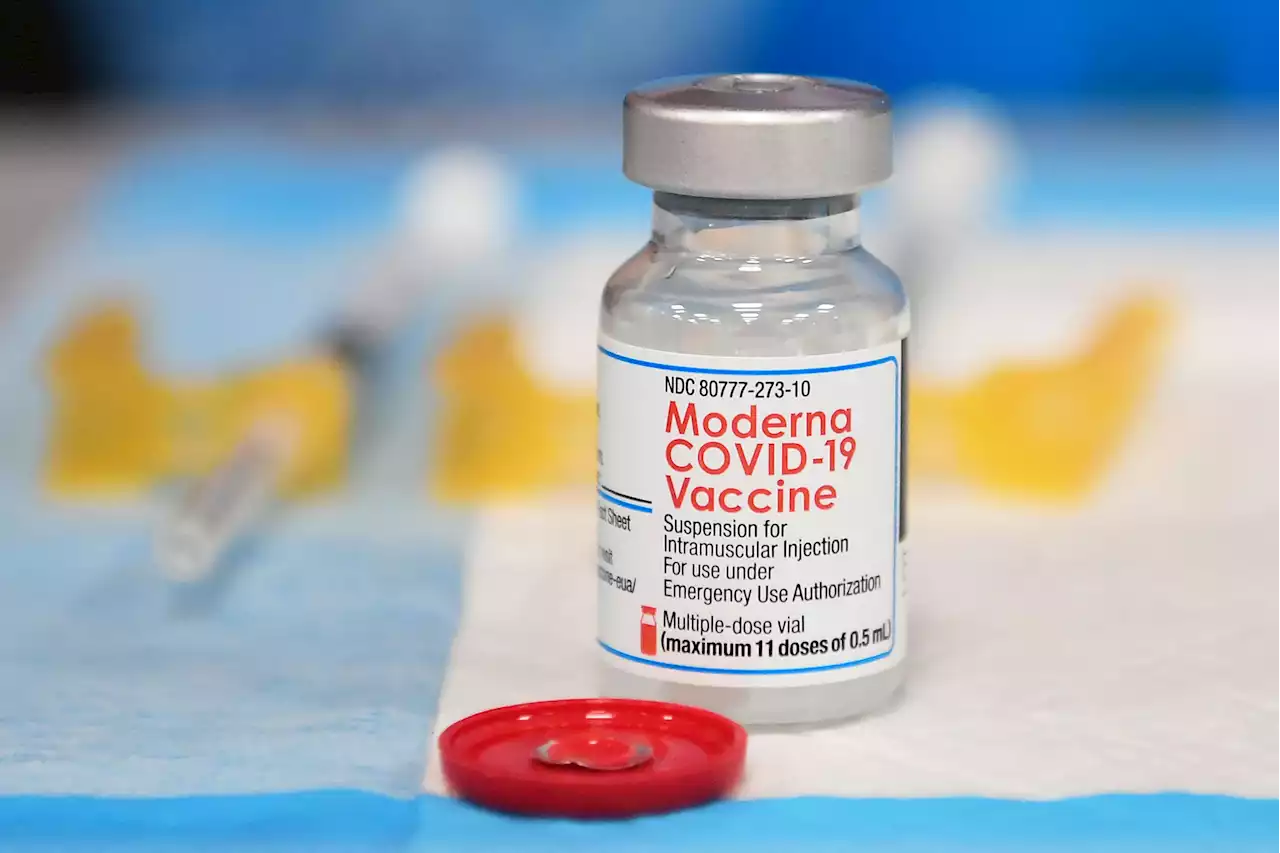 FDA Advisers Back Moderna's COVID-19 Vaccine for Older KidsA government advisory panel Tuesday endorsed a second brand of COVID-19 vaccine for school-age children and teens. The Food and Drug Administration’s outside experts voted unanimously that Moderna’s vaccine is safe and effective enough to give kids ages 6 to 17. If the FDA agrees, it would become the second option for those children, joining Pfizer’s vaccine. The same FDA…
FDA Advisers Back Moderna's COVID-19 Vaccine for Older KidsA government advisory panel Tuesday endorsed a second brand of COVID-19 vaccine for school-age children and teens. The Food and Drug Administration’s outside experts voted unanimously that Moderna’s vaccine is safe and effective enough to give kids ages 6 to 17. If the FDA agrees, it would become the second option for those children, joining Pfizer’s vaccine. The same FDA…
Baca lebih lajut »
 FDA advisers back Moderna's COVID-19 vaccine for older kidsA government advisory panel has endorsed a second brand of COVID-19 vaccine for school-age children and teens.
FDA advisers back Moderna's COVID-19 vaccine for older kidsA government advisory panel has endorsed a second brand of COVID-19 vaccine for school-age children and teens.
Baca lebih lajut »
 FDA advisers back Moderna's COVID-19 vaccine for kids ages 6 and upThe Food and Drug Administration's outside experts voted unanimously that Moderna's vaccine is safe and effective enough to give kids ages 6 to 17. If the FDA agrees, it would become the second option for those children, joining Pfizer's vaccine.
FDA advisers back Moderna's COVID-19 vaccine for kids ages 6 and upThe Food and Drug Administration's outside experts voted unanimously that Moderna's vaccine is safe and effective enough to give kids ages 6 to 17. If the FDA agrees, it would become the second option for those children, joining Pfizer's vaccine.
Baca lebih lajut »
 FDA says Pfizer COVID-19 vaccine appears effective for children under 5The FDA said Pfizer's coronavirus vaccine appears to be safe for children younger than 5, the only age group not yet eligible for vaccination in the U.S.
FDA says Pfizer COVID-19 vaccine appears effective for children under 5The FDA said Pfizer's coronavirus vaccine appears to be safe for children younger than 5, the only age group not yet eligible for vaccination in the U.S.
Baca lebih lajut »
