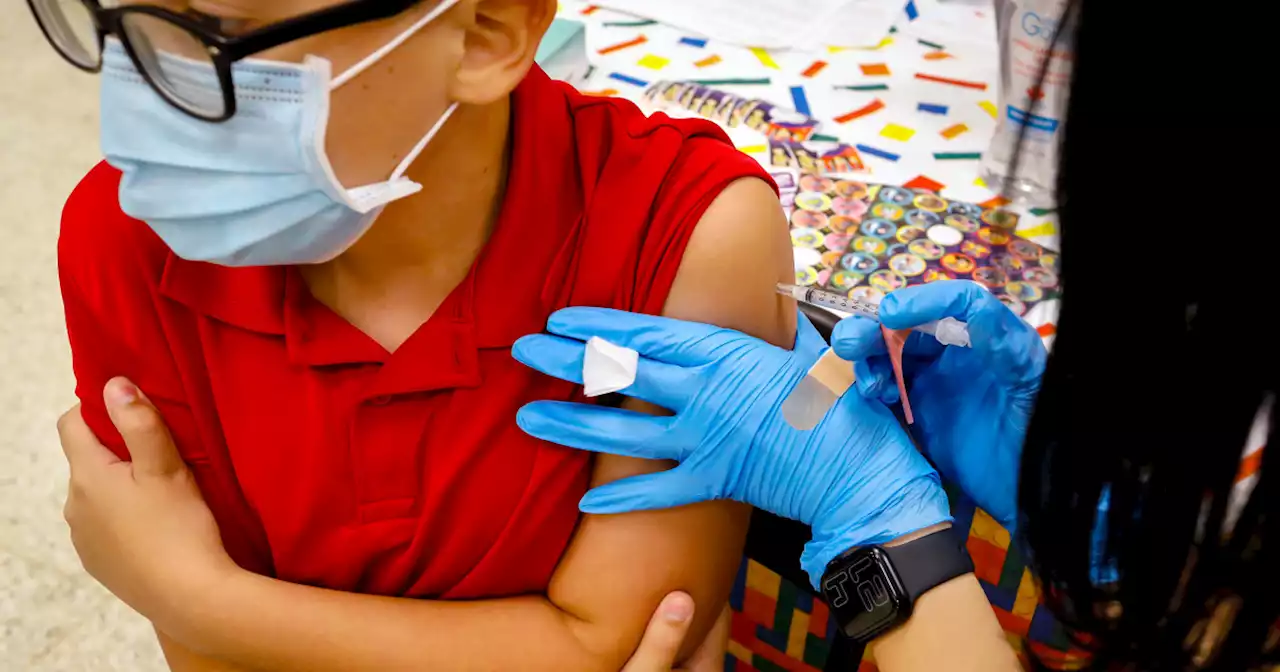
NEW: FDA authorizes booster dose of the Pfizer Covid-19 vaccine for children ages 5 to 11.
Children in the age group can get a booster shot at least five months after they’ve received the primary two-dose series, the FDA said in a statement.The booster shot is 10 micrograms, the same dosage as the primary series for the age group and a third of the dosage given to people ages 12 and up.
Less than a third of the 28 million 5 -to-11-year-old children in the United States have received two doses of a Covid vaccine, according to data from the CDC. , which appear to be more adept than previous strains at sidestepping immunity from vaccinations or prior infection.found a booster dose of the Pfizer vaccine in children 5-11 raised antibody levels against the original strain of the coronavirus as well as the omicron variant. The results did not show how long the antibodies remain elevated, though higher antibody levels from boosters in adults usually persist for about four months.
Two doses were very good at protecting against mild disease from the delta variant “but not very good against omicron,” Offit said.a third Pfizer dose for children 5-11 with compromised immune systems as part of their three-dose primary series.
Indonesia Berita Terbaru, Indonesia Berita utama
Similar News:Anda juga dapat membaca berita serupa dengan ini yang kami kumpulkan dari sumber berita lain.
 Without COVID-19 vaccines, death toll would be much higher: Pfizer analysisIn the wake of the tragic milestone of 1 million official COVID-19 deaths in the U.S., a new analysis shows that without vaccines, the virus would have likely claimed more than 100,000 additional lives in 2021.
Without COVID-19 vaccines, death toll would be much higher: Pfizer analysisIn the wake of the tragic milestone of 1 million official COVID-19 deaths in the U.S., a new analysis shows that without vaccines, the virus would have likely claimed more than 100,000 additional lives in 2021.
Baca lebih lajut »
 FDA Limits Use of J&J COVID Vaccine Over Blood Clot RiskThe FDA is limiting who can receive the Johnson & Johnson COVID-19 vaccine because of concerns about the risk of a rare blood clotting condition.
FDA Limits Use of J&J COVID Vaccine Over Blood Clot RiskThe FDA is limiting who can receive the Johnson & Johnson COVID-19 vaccine because of concerns about the risk of a rare blood clotting condition.
Baca lebih lajut »
 FDA authorizes first non-prescription test for COVID-19, flu, RSVNEW: The FDA has authorized the first non-prescription COVID-19 test that can also detect the flu and RSV.
FDA authorizes first non-prescription test for COVID-19, flu, RSVNEW: The FDA has authorized the first non-prescription COVID-19 test that can also detect the flu and RSV.
Baca lebih lajut »
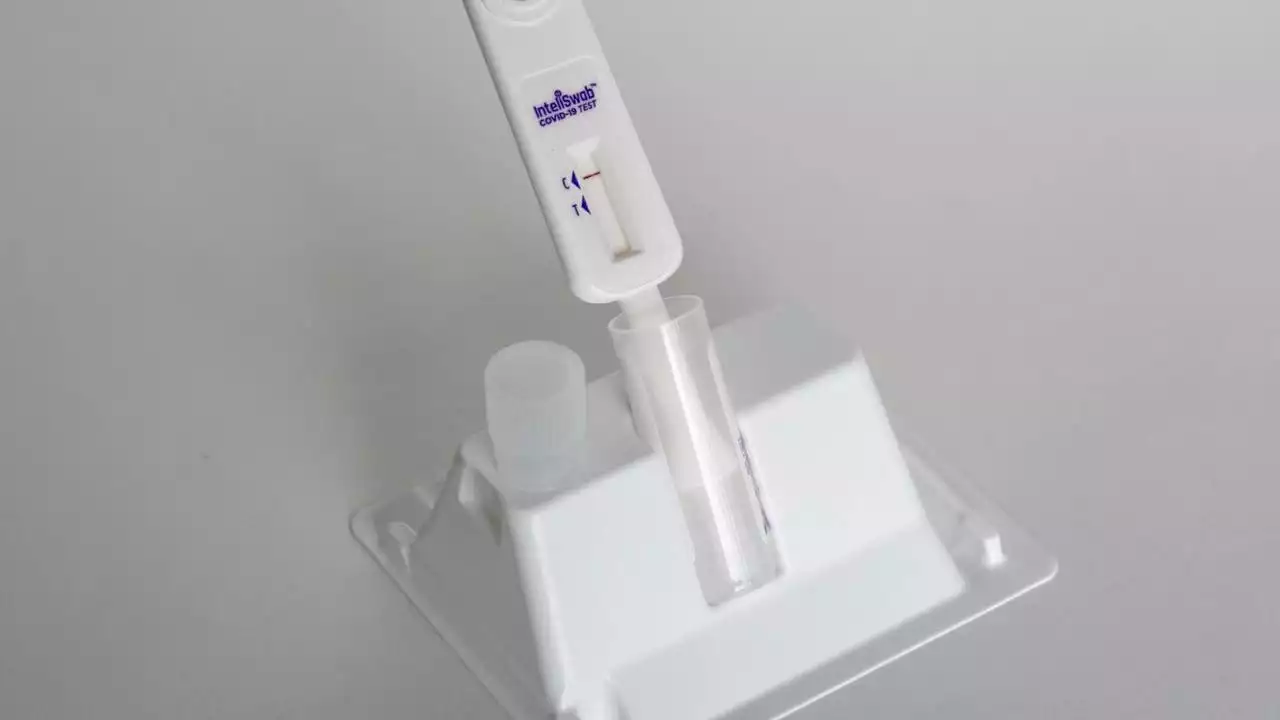 FDA approves first non-prescription COVID test that also detects flu, RSVThe U.S. Food and Drug Administration issued its approval Monday of the first non-prescription at-home COVID-19 test that also detects the flu.
FDA approves first non-prescription COVID test that also detects flu, RSVThe U.S. Food and Drug Administration issued its approval Monday of the first non-prescription at-home COVID-19 test that also detects the flu.
Baca lebih lajut »
 FDA Authorizes Nonprescription Test for Covid-19, Flu and RSVThe FDA authorized the first nonprescription test for Covid-19, influenza and RSV, in another step toward increasing patient access to tests for Covid-19 and other conditions
FDA Authorizes Nonprescription Test for Covid-19, Flu and RSVThe FDA authorized the first nonprescription test for Covid-19, influenza and RSV, in another step toward increasing patient access to tests for Covid-19 and other conditions
Baca lebih lajut »
 FDA: fluvoxamine doesn’t treat COVID-19 — and here’s 27 pages whyThat transparency isn’t normal.
FDA: fluvoxamine doesn’t treat COVID-19 — and here’s 27 pages whyThat transparency isn’t normal.
Baca lebih lajut »