The FDA is limiting who can receive the Johnson & Johnson COVID-19 vaccine because of concerns about the risk of a rare blood clotting condition.
issued Thursday, the FDA said the J&J vaccine should only be given to people 18 and older who don’t have access to other vaccines or for whom other vaccines are not clinically appropriate. People 18 and older can also get the J&J vaccine if they choose to because they wouldn’t otherwise receive any vaccine, the FDA said.
syndrome , 1 or 2 weeks after people received the J&J vaccine. The finding “warrants limiting the authorized use of the vaccine,” the FDA said.response in the United States and across the global community,” Peter Marks, MD, director of the FDA’s Center for Biologics Evaluation and Research, said in the statement.
“Our action reflects our updated analysis of the risk of TTS following administration of this vaccine and limits the use of the vaccine to certain individuals.”says 16.9 million people are fully vaccinated with the J&J vaccine, compared to 76.5 million with Moderna and 126.3 million with Pfizer. Through March 18, the CDC and FDA have detected 60 confirmed cases of TTS, including nine fatal cases,The J&J vaccine was granted emergency authorization in February 2021. Health authorities hoped it would help spread vaccines across the nation because it only required one initial dose and didn’t need to be stored at extremely cold temperatures, unlike the two-dose Pfizer and Moderna vaccines.
But 2 months after authorization, the government paused its use for 10 days because of reports of TTS. Last December, the CDC’s
Indonesia Berita Terbaru, Indonesia Berita utama
Similar News:Anda juga dapat membaca berita serupa dengan ini yang kami kumpulkan dari sumber berita lain.
 FDA authorizes underwear that protects against STD'sThe resident ladies of Girl Talk have an interesting topic on the table: underwear that can prevent sexually transmitted infections. The FDA has authorized a company's specially designed underwear. But are they practical?
FDA authorizes underwear that protects against STD'sThe resident ladies of Girl Talk have an interesting topic on the table: underwear that can prevent sexually transmitted infections. The FDA has authorized a company's specially designed underwear. But are they practical?
Baca lebih lajut »
 Baby formula shortage: White House directs FDA to import more baby formula amid nationwide shortageA baby formula shortage in the United States is driving parents to swap, sell and offer leftover supplies to each other, as President Joe Biden spoke with manufacturers and retailers Thursday about the plight facing families.
Baby formula shortage: White House directs FDA to import more baby formula amid nationwide shortageA baby formula shortage in the United States is driving parents to swap, sell and offer leftover supplies to each other, as President Joe Biden spoke with manufacturers and retailers Thursday about the plight facing families.
Baca lebih lajut »
 4 Air Force cadets may not graduate for refusing COVID-19 vaccineIt's the only military academy, so far, where cadets may face such penalties.
4 Air Force cadets may not graduate for refusing COVID-19 vaccineIt's the only military academy, so far, where cadets may face such penalties.
Baca lebih lajut »
 4 Air Force cadets may not graduate due to vaccine refusalIt's the only military academy, so far, where cadets may not graduate for refusing the COVID-19 vaccine.
4 Air Force cadets may not graduate due to vaccine refusalIt's the only military academy, so far, where cadets may not graduate for refusing the COVID-19 vaccine.
Baca lebih lajut »
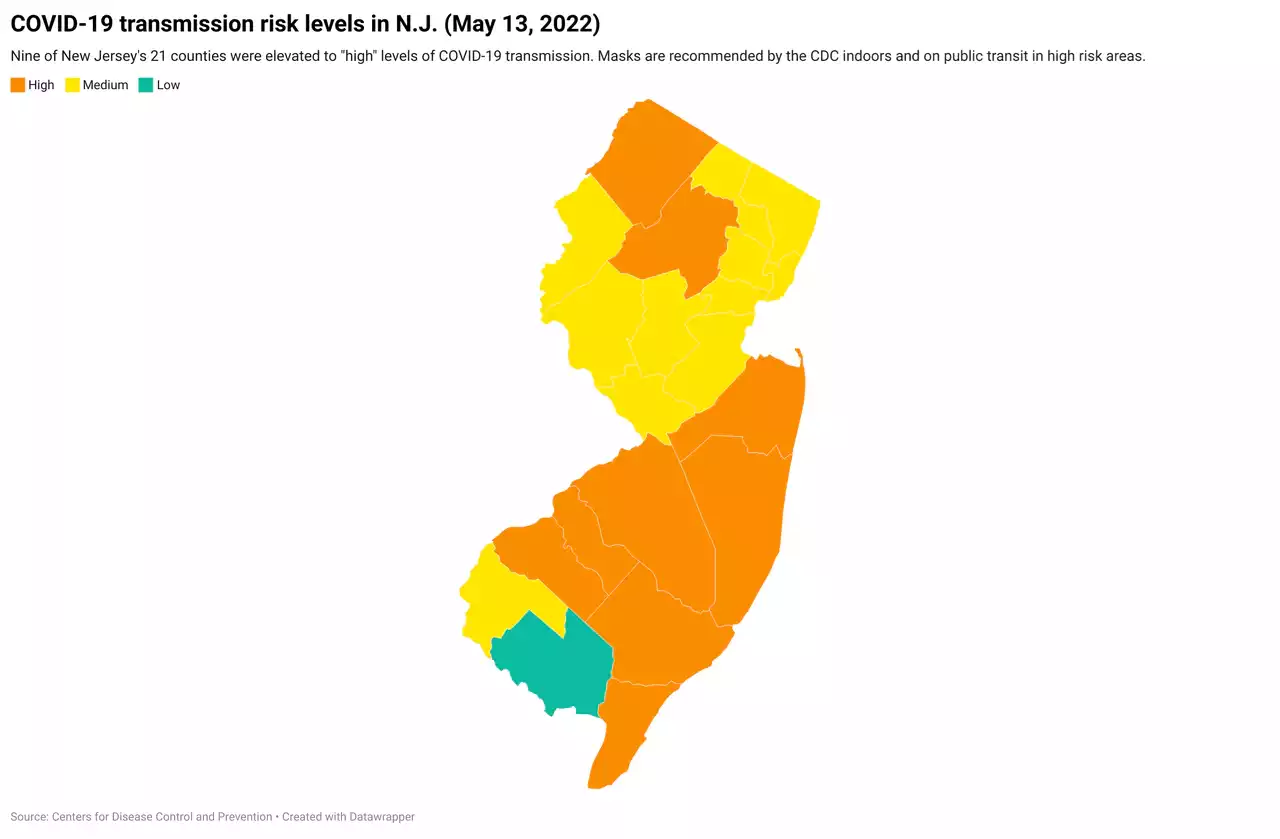 Masks recommended indoors in 9 N.J. counties with ‘high’ COVID risk, CDC saysThe CDC elevated 9 New Jersey counties to 'high' COVID transmission risk, the first time any county was deemed high under new federal rankings.
Masks recommended indoors in 9 N.J. counties with ‘high’ COVID risk, CDC saysThe CDC elevated 9 New Jersey counties to 'high' COVID transmission risk, the first time any county was deemed high under new federal rankings.
Baca lebih lajut »