JUST IN: FDA authorizes COVID vaccine for children under 5, CDC review next abc15
The Food and Drug Administration gave its authorization on Friday for both Pfizer’s and Modern’s COVID-19 vaccines for children under age 5.
On Wednesday, an FDA advisory panel unanimously found both vaccines to be “safe and effective” for children as young as 6 months old.
Indonesia Berita Terbaru, Indonesia Berita utama
Similar News:Anda juga dapat membaca berita serupa dengan ini yang kami kumpulkan dari sumber berita lain.
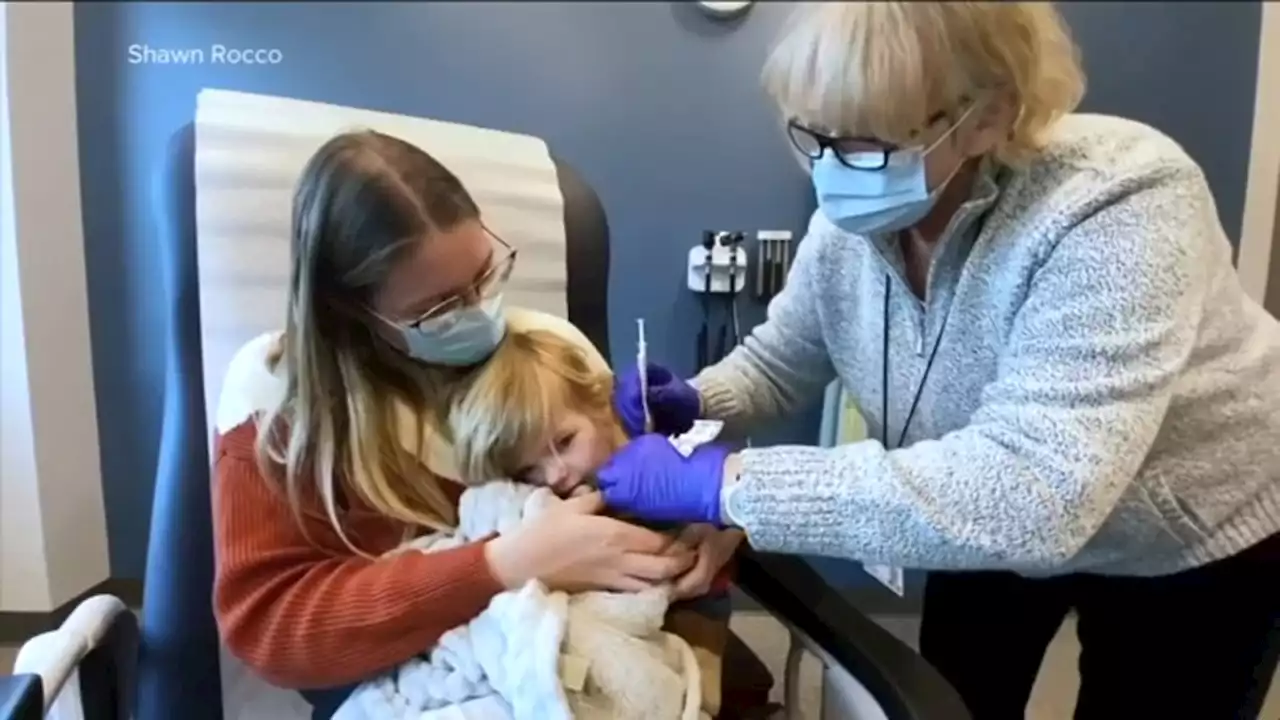
 CDC panel meeting on COVID-19 vaccine for younger kidsThe next steps towards approving COVID vaccines for young children are underway and in the hands of the CDC.
CDC panel meeting on COVID-19 vaccine for younger kidsThe next steps towards approving COVID vaccines for young children are underway and in the hands of the CDC.
Baca lebih lajut »
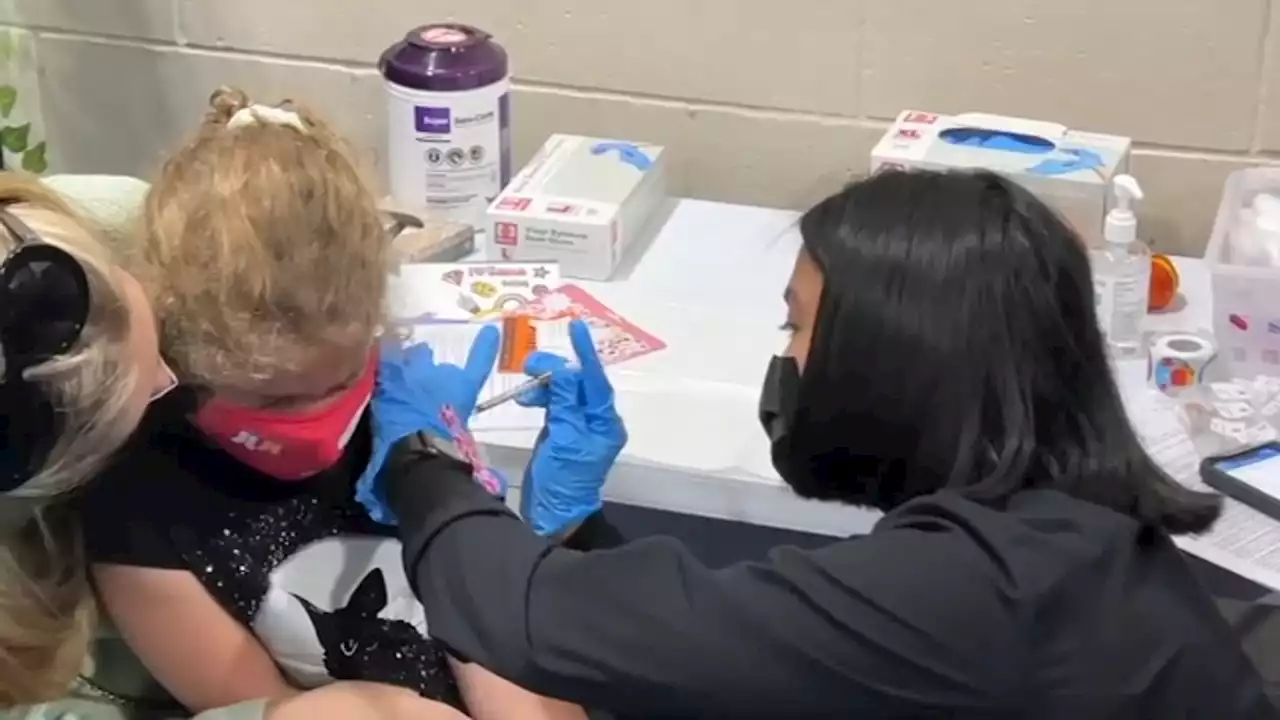
 COVID-19 vaccine for younger kids could be available next week, waiting on CDC approvalThe Food and Drug Administration's vaccine advisers gave a thumbs-up to vaccines from Moderna and Pfizer for the littlest kids.
COVID-19 vaccine for younger kids could be available next week, waiting on CDC approvalThe Food and Drug Administration's vaccine advisers gave a thumbs-up to vaccines from Moderna and Pfizer for the littlest kids.
Baca lebih lajut »
 COVID-19 vaccine for younger kids could be available next week, waiting on CDC approvalThe Food and Drug Administration's vaccine advisers gave a thumbs-up to vaccines from Moderna and Pfizer for the littlest kids.
COVID-19 vaccine for younger kids could be available next week, waiting on CDC approvalThe Food and Drug Administration's vaccine advisers gave a thumbs-up to vaccines from Moderna and Pfizer for the littlest kids.
Baca lebih lajut »
 FDA moves closer to authorizing COVID-19 shots for kids under 5If all the regulatory steps are cleared, the shots should be available next week.
FDA moves closer to authorizing COVID-19 shots for kids under 5If all the regulatory steps are cleared, the shots should be available next week.
Baca lebih lajut »
 'Finally': Parents react to FDA endorsing COVID vaccines for kids under 5FDA advisers recommend that both Moderna and Pfizer COVID vaccines for kids under 5 be authorized for emergency use. Parents are both angry and relieved.
'Finally': Parents react to FDA endorsing COVID vaccines for kids under 5FDA advisers recommend that both Moderna and Pfizer COVID vaccines for kids under 5 be authorized for emergency use. Parents are both angry and relieved.
Baca lebih lajut »
