Infectious disease experts explain what parents need to know.
, a pediatrician at Cleveland Clinic, tells SELF. She says it’s helpful to think of the booster as “another layer of protection” for children who have received the primary vaccination series.
In addition to keeping children safe from severe COVID-19, the booster dose could serve the community as a whole. “Getting the COVID-19 [booster] protects children and those around them—particularly those like grandparents or older relatives who may be more vulnerable to COVID-19,” Dr. Giuliano says. “The more people who are vaccinated, including children, the better chance we will have of slowing the spread of COVID-19.
The booster dose is especially important for children who may be spending significant amounts of time with unvaccinated individuals, such as in classrooms or sporting events. “In areas with lower vaccination rates, the booster is even more important as the spread of disease will likely be greater,” Dr. Giuliano adds.
That said, a booster dose won’t necessarily prevent infection in every single case, as we’ve seen over the past few months since omicron emerged, says Dr. Russo. If parents are unsure about whether they should vaccinate their children, they should turn to their health-care providers, experts say. “It’s understandable that parents may have questions or concerns about COVID-19 vaccination and boosting for their children,” Dr. Giuliano says.
Part of the reason the booster dose was authorized, Dr. Russo adds, comes down to what scientists and doctors are currently seeing: a large number of people infected with omicron even after vaccination and a booster dose. “We are seeing another increase in COVID-19 cases across the country,” Dr. Giuliano adds.
Indonesia Berita Terbaru, Indonesia Berita utama
Similar News:Anda juga dapat membaca berita serupa dengan ini yang kami kumpulkan dari sumber berita lain.
 FDA approves Pfizer COVID-19 booster for children ages 5 to 11Pfizer’s COVID-19 booster shot for U.S. kids ages 5 to 11 has been approved by the FDA. Next, it will need the CDC’s final sign-off.
FDA approves Pfizer COVID-19 booster for children ages 5 to 11Pfizer’s COVID-19 booster shot for U.S. kids ages 5 to 11 has been approved by the FDA. Next, it will need the CDC’s final sign-off.
Baca lebih lajut »
 FDA approves Pfizer COVID booster shots for kids 5 to 11The FDA has granted emergency use authorization for a booster dose of Pfizer/BioNTech's COVID-19 vaccine for children ages 5 to 11.
FDA approves Pfizer COVID booster shots for kids 5 to 11The FDA has granted emergency use authorization for a booster dose of Pfizer/BioNTech's COVID-19 vaccine for children ages 5 to 11.
Baca lebih lajut »
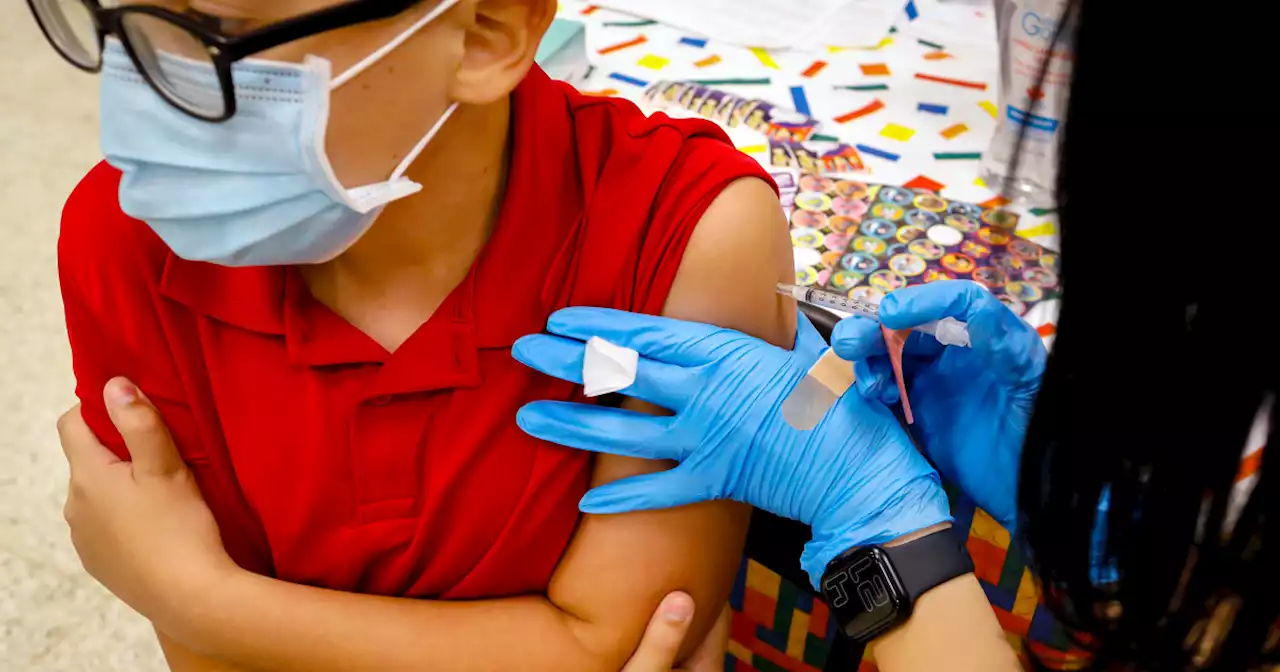 FDA authorizes Pfizer Covid booster for children 5 to 11 years oldA study in February found that two doses of the vaccine offered little protection against the omicron variant.
FDA authorizes Pfizer Covid booster for children 5 to 11 years oldA study in February found that two doses of the vaccine offered little protection against the omicron variant.
Baca lebih lajut »
 FDA Authorizes Pfizer Covid Booster Dose for Kids Ages 5 to 11 Years OldThe Food and Drug Administration on Tuesday authorized a Pfizer booster dose for children ages 5 through 11 years old at least five months after they complete their two-dose primary series. Dr. Peter Marks, head of the FDA division responsible for vaccines, said data increasingly shows that the protection provided by two shots wanes off over time. The FDA determined…
FDA Authorizes Pfizer Covid Booster Dose for Kids Ages 5 to 11 Years OldThe Food and Drug Administration on Tuesday authorized a Pfizer booster dose for children ages 5 through 11 years old at least five months after they complete their two-dose primary series. Dr. Peter Marks, head of the FDA division responsible for vaccines, said data increasingly shows that the protection provided by two shots wanes off over time. The FDA determined…
Baca lebih lajut »
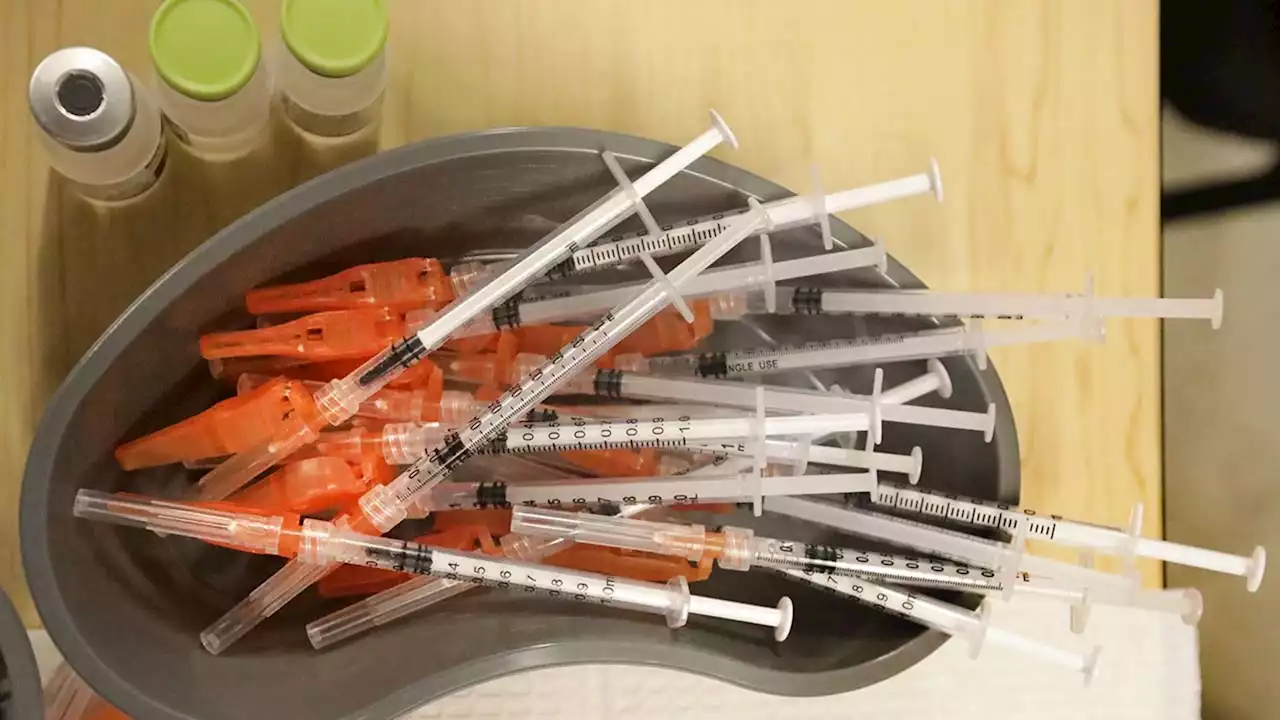 FDA authorizes Pfizer COVID-19 booster shots for children ages 5 to 11The FDA has granted emergency use authorization for a booster dose of Pfizer/BioNTech's COVID-19 vaccine for children ages 5 to 11 at least five months after completion of the primary vaccine series.
FDA authorizes Pfizer COVID-19 booster shots for children ages 5 to 11The FDA has granted emergency use authorization for a booster dose of Pfizer/BioNTech's COVID-19 vaccine for children ages 5 to 11 at least five months after completion of the primary vaccine series.
Baca lebih lajut »
 FDA authorizes Pfizer COVID-19 booster shots for children ages 5 to 11The FDA has granted emergency use authorization for a booster dose of Pfizer/BioNTech's COVID-19 vaccine for children ages 5 to 11 at least five months after completion of the primary vaccine series.
FDA authorizes Pfizer COVID-19 booster shots for children ages 5 to 11The FDA has granted emergency use authorization for a booster dose of Pfizer/BioNTech's COVID-19 vaccine for children ages 5 to 11 at least five months after completion of the primary vaccine series.
Baca lebih lajut »
