After two years at the front of the line for COVID-19 vaccines and treatments, the U.S. could soon have to begin taking a number.
White House press secretary Jen Psaki speaks at a press briefing at the White House in Washington, Monday, April 25, 2022. – For much of the past two years, America has been first in line for COVID-19 vaccines and treatments. Now, as drugmakers develop the next generation of therapies, the White House is warning that if Congress doesn’t act urgently the U.S. will have to take a number.
The White House this week is mounting a push for doctors to get less stingy about prescribing the antiviral pill Paxlovid, which was initially rationed for those at the highest risk for severe outcomes from COVID-19 but is now more widely available. A 20 million-dose order placed last year by the government helped boost manufacturing capacity.
Complicating matters further are the long lead times to manufacture the antiviral and antibody treatments. Paxlovid takes about six months to produce, and monoclonal antibody treatments used to treat COVID-19 and prevent serious disease in the immunocompromised take similarly long, meaning the U.S. is running out of time to replenish its stockpile before the end of the year.
Moderna and Pfizer both are testing what scientists call “bivalent” shots — a mix of each company’s original vaccine and an omicron-targeted version — with Moderna announcing last week it hopes to have its version ready this fall. An election-year vote to extend that order would be perilous for Democrats, and many hope no such vote occurs. Many say privately they hope Biden will keep the immigration curbs in place or that a court will postpone the rules’ termination, but Republicans could well force a vote anyway.
Indonesia Berita Terbaru, Indonesia Berita utama
Similar News:Anda juga dapat membaca berita serupa dengan ini yang kami kumpulkan dari sumber berita lain.
 FDA approves COVID drug for children, China deals with uphill COVID battle, and more COVID newsThe US Food and Drug Administration announced Monday that it has expanded approval of the COVID-19 drug remdesivir to treat patients as young as 28 days old. Here's that and more COVID news.
FDA approves COVID drug for children, China deals with uphill COVID battle, and more COVID newsThe US Food and Drug Administration announced Monday that it has expanded approval of the COVID-19 drug remdesivir to treat patients as young as 28 days old. Here's that and more COVID news.
Baca lebih lajut »
 White House urges more funding for COVID treatmentsThe Senate last month closed in on smaller $10 billion package focused on domestic needs. But even that deal fell apart as lawmakers objected to an announcement from the Centers for Disease Control…
White House urges more funding for COVID treatmentsThe Senate last month closed in on smaller $10 billion package focused on domestic needs. But even that deal fell apart as lawmakers objected to an announcement from the Centers for Disease Control…
Baca lebih lajut »
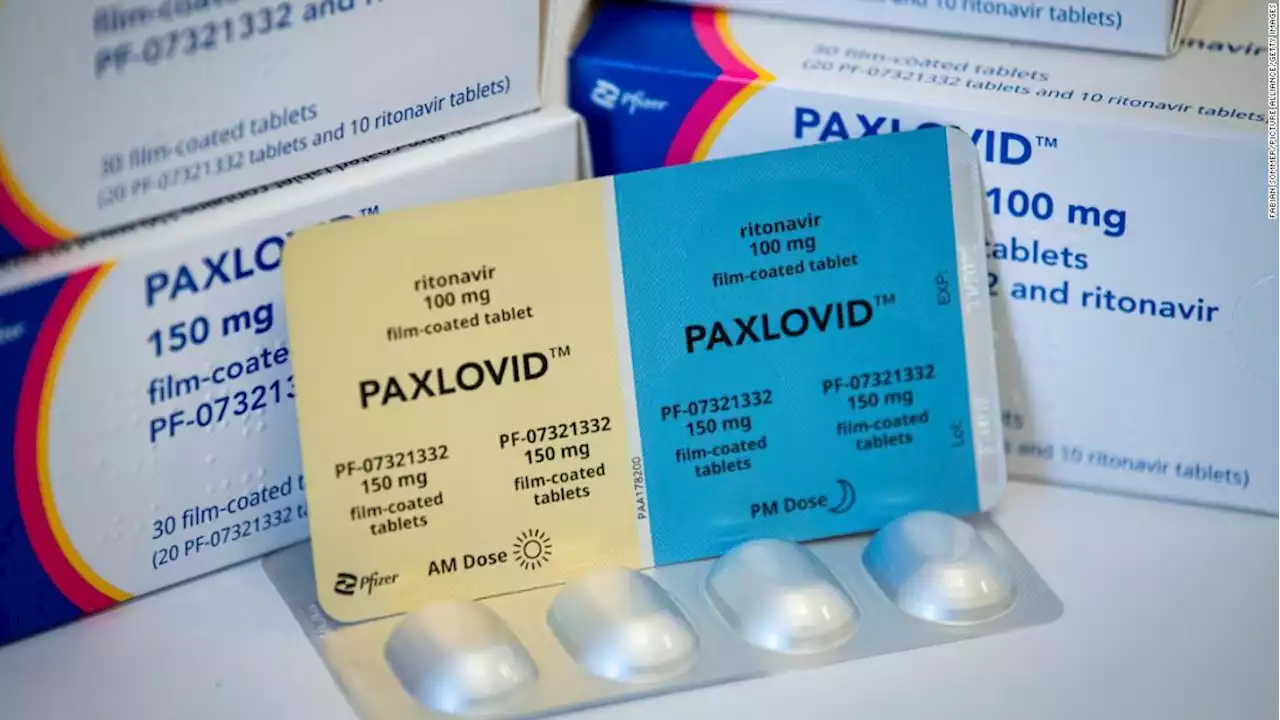 White House working to make Pfizer's Covid-19 antiviral pill more 'widely available'The Biden administration is set to unveil new details and announcements Monday on Paxlovid, Pfizer's antiviral Covid-19 pill, a White House official tells CNN.
White House working to make Pfizer's Covid-19 antiviral pill more 'widely available'The Biden administration is set to unveil new details and announcements Monday on Paxlovid, Pfizer's antiviral Covid-19 pill, a White House official tells CNN.
Baca lebih lajut »
 House Republicans say EcoHealth Alliance concealed COVID-19 research data in effort to preserve fundingHouse Energy and Commerce Committee Republicans are calling for further investigation into EcoHealth Alliance and its president after the organization allegedly withheld certain COVID-19 research data from peer reviewers in an effort to retain funding from the National Institutes of Health.
House Republicans say EcoHealth Alliance concealed COVID-19 research data in effort to preserve fundingHouse Energy and Commerce Committee Republicans are calling for further investigation into EcoHealth Alliance and its president after the organization allegedly withheld certain COVID-19 research data from peer reviewers in an effort to retain funding from the National Institutes of Health.
Baca lebih lajut »
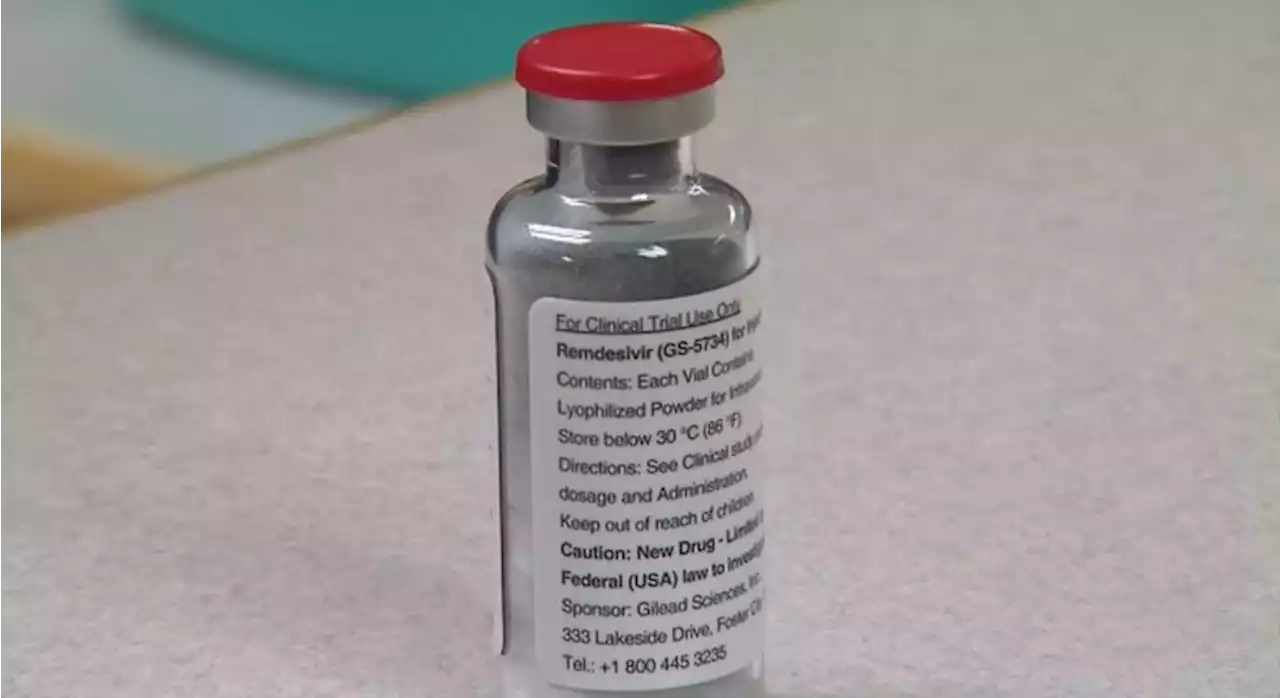 FDA approves Remdesivir to treat COVID in young childrenThe antiviral drug is given as an injection. It was previously approved for patients 12 years of age and older.
FDA approves Remdesivir to treat COVID in young childrenThe antiviral drug is given as an injection. It was previously approved for patients 12 years of age and older.
Baca lebih lajut »
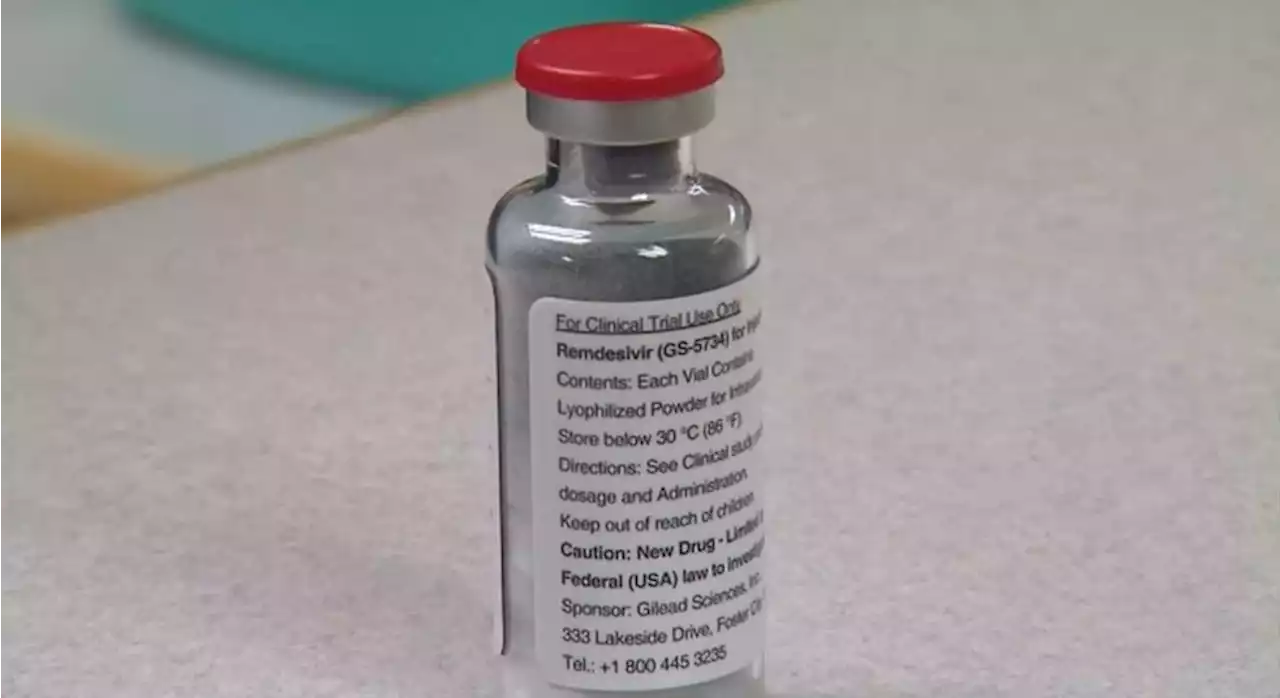 FDA approves Remdesivir to treat COVID in young childrenThe antiviral drug is given as an injection. It was previously approved for patients 12 years of age and older.
FDA approves Remdesivir to treat COVID in young childrenThe antiviral drug is given as an injection. It was previously approved for patients 12 years of age and older.
Baca lebih lajut »
