The Novavax vaccine just received emergency use authorization from the FDA.
citizens with another option."From a scientific standpoint, I think the fact that we have another tool to be able to mix and match with other vaccines to come up with theHow does the Novavax vaccine differ from other COVID vaccines?
"The chief difference that this is a protein-based vaccine, as Moderna and Pfizer vaccines are mRNA-based and the Johnson & Johnson uses a different virus to deliver the spike protein of SARS-CoV-2," Dr. Adalja says. Novavax is a vaccine that delivers the spike protein"directly in the form of a protein," which produces an immune response in the body that will protect against a future interaction with the virus.
That being said, because the Novavax vaccine uses a spike protein based on the"ancestral" strain of the virus ,"it will likely have difficulty in preventinginfections from Omicron variants, just like the current other current vaccines," Dr. Adalja says. But one thing that differs in Novavax is that the spike protein is coupled with an adjuvant,"a substance that stimulates the immune system," Dr.
Common side effects are similar to other vaccines, including fatigue, tenderness or pain at the injection site, headache, and muscle pain. There has also been some concern over the vaccine's ability to cause myocarditis and pericarditis.
Indonesia Berita Terbaru, Indonesia Berita utama
Similar News:Anda juga dapat membaca berita serupa dengan ini yang kami kumpulkan dari sumber berita lain.
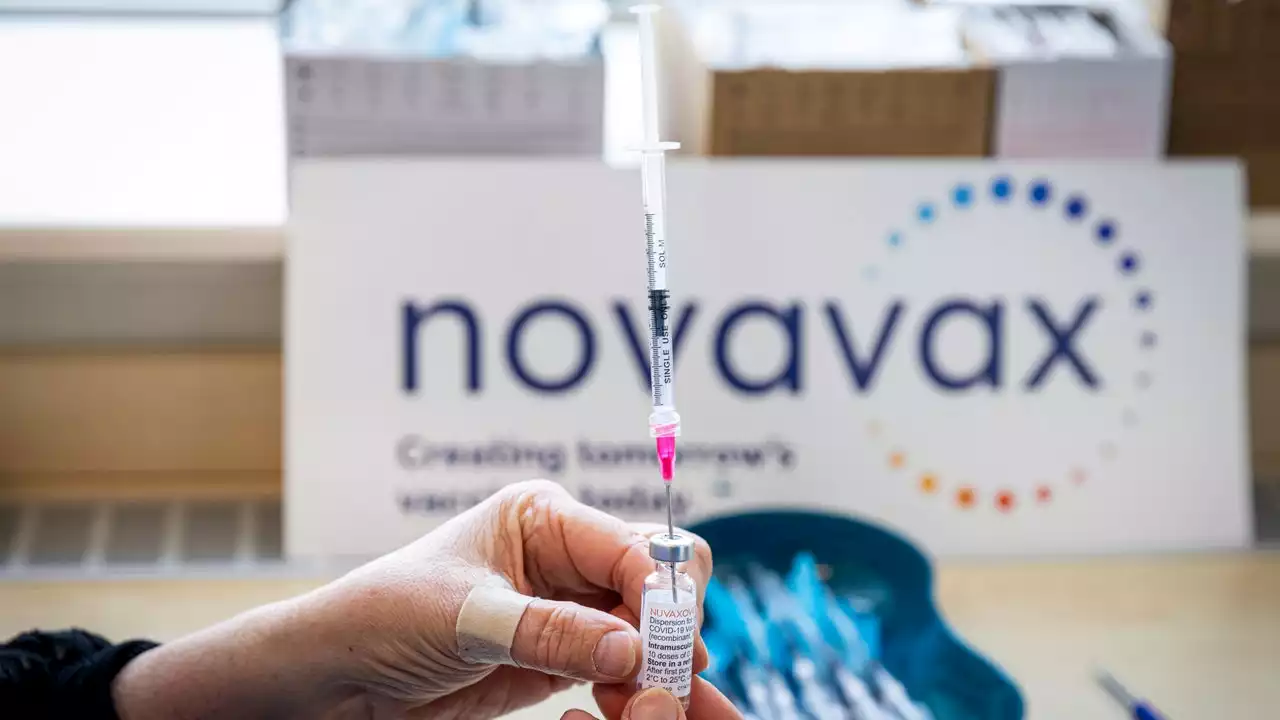 Novavax COVID-19 vaccine: FDA authorizes fourth shot for adultsNovavax makes a more traditional type of shot than the three other COVID-19 vaccines available for use in the U.S. -- and one that’s already available in Europe and multiple other countries.
Novavax COVID-19 vaccine: FDA authorizes fourth shot for adultsNovavax makes a more traditional type of shot than the three other COVID-19 vaccines available for use in the U.S. -- and one that’s already available in Europe and multiple other countries.
Baca lebih lajut »
 Novavax Covid-19 vaccine wins FDA authorizationThe FDA granted emergency use authorization to Novavax’s Covid-19 vaccine. In a trial of more than 26,000 adults, 2 doses of the Novavax Covid vaccine were over 90% effective at preventing symptomatic disease and presented no serious side effects.
Novavax Covid-19 vaccine wins FDA authorizationThe FDA granted emergency use authorization to Novavax’s Covid-19 vaccine. In a trial of more than 26,000 adults, 2 doses of the Novavax Covid vaccine were over 90% effective at preventing symptomatic disease and presented no serious side effects.
Baca lebih lajut »
 FDA authorizes Novavax COVID-19 vaccineThe FDA on Wednesday issued an emergency use authorization for Novavax's COVID-19 vaccine for adults 18 and older.
FDA authorizes Novavax COVID-19 vaccineThe FDA on Wednesday issued an emergency use authorization for Novavax's COVID-19 vaccine for adults 18 and older.
Baca lebih lajut »
 FDA Clears New COVID-19 Vaccine Option From Novavax for AdultsNearly a quarter of American adults still haven't gotten their primary vaccinations even this late in the COVID pandemic, and experts expect at least some of them to roll up their sleeves for a more conventional option — a protein-based vaccine.
FDA Clears New COVID-19 Vaccine Option From Novavax for AdultsNearly a quarter of American adults still haven't gotten their primary vaccinations even this late in the COVID pandemic, and experts expect at least some of them to roll up their sleeves for a more conventional option — a protein-based vaccine.
Baca lebih lajut »
 Novavax COVID-19 vaccine: FDA authorizes new shot for adultsNovavax makes a more traditional type of shot than the three other COVID-19 vaccines available for use in the U.S. -- and one that’s already available in Europe and multiple other countries.
Novavax COVID-19 vaccine: FDA authorizes new shot for adultsNovavax makes a more traditional type of shot than the three other COVID-19 vaccines available for use in the U.S. -- and one that’s already available in Europe and multiple other countries.
Baca lebih lajut »
 FDA Authorizes Novavax Covid Vaccine for Adults as the First New Shots in U.S. in More Than a YearFDA authorization of Novavax’s vaccine was delayed for weeks as the agency reviewed changes to the company’s manufacturing process.
FDA Authorizes Novavax Covid Vaccine for Adults as the First New Shots in U.S. in More Than a YearFDA authorization of Novavax’s vaccine was delayed for weeks as the agency reviewed changes to the company’s manufacturing process.
Baca lebih lajut »