FDA grants approval on RSV vaccine.
CLEVELAND, Ohio - The U.S. Food and Drug Administration approved the first RSV vaccine on Monday, clearing the way for usage to prevent RSV in infants from birth to six months.
The vaccine, called Abrysvo, is approved for use at 32 to 36 weeks during pregnancy. The single dose vaccine comes a few months after its approval for people 60 and older. RSV cases have been rising over the last few years, causing respiratory infections in people of all ages, but more frequently in infants. RSV typically is seasonal, appearing prior to flu season and peaking in the winter. While RSV can be problematic to anyone,“in infants and children, the risk of RSV-associated LRTD is highest during the first year of life. According to the Centers for Disease Control and Prevention, RSV is the leading cause of infant hospitalization in the U.S”.
The study that ultimately led to the approval of Abrysvo showed that the vaccine was 81.8% successful in reducing the risk for RSV within 90 days of birth, and 69.4% within 180 days of birth. In a subgroup of women who were 32 through 36 weeks pregnant, severe risk of RSV was reduced by 91.1% within 90 days of birth.
Indonesia Berita Terbaru, Indonesia Berita utama
Similar News:Anda juga dapat membaca berita serupa dengan ini yang kami kumpulkan dari sumber berita lain.
 FDA approves first RSV vaccine for pregnancy to protect newbornsExpectant parents could soon have another new option this fall to protect their newborns from RSV, the most common cause of hospitalization in American infants.
FDA approves first RSV vaccine for pregnancy to protect newbornsExpectant parents could soon have another new option this fall to protect their newborns from RSV, the most common cause of hospitalization in American infants.
Baca lebih lajut »
 US FDA approves Pfizer's maternal RSV vaccine to protect infantsThe U.S. Food and Drug Administration on Monday approved Pfizer's respiratory syncytial virus (RSV) vaccine for use in women during the middle of the third trimester of pregnancy to protect their babies.
US FDA approves Pfizer's maternal RSV vaccine to protect infantsThe U.S. Food and Drug Administration on Monday approved Pfizer's respiratory syncytial virus (RSV) vaccine for use in women during the middle of the third trimester of pregnancy to protect their babies.
Baca lebih lajut »
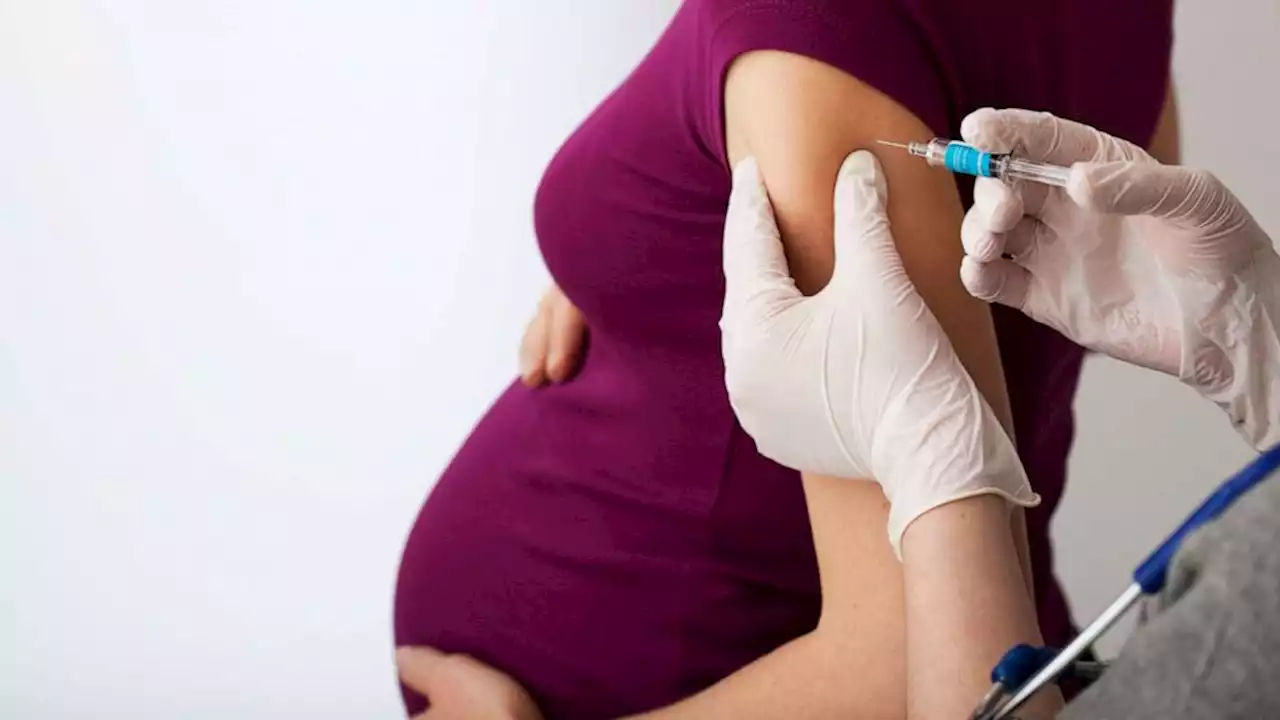 FDA approves 1st maternal RSV vaccine to help protect infantsThe U.S. Food and Drug Administration has approved the first maternal vaccine intended to help protect newborns against respiratory syncytial virus (RSV).
FDA approves 1st maternal RSV vaccine to help protect infantsThe U.S. Food and Drug Administration has approved the first maternal vaccine intended to help protect newborns against respiratory syncytial virus (RSV).
Baca lebih lajut »
 FDA approves Pfizer maternal RSV vaccine for infantsPfizer hopes that its respiratory syncytial virus vaccine will be available to the public by the end of October or the beginning of November.
FDA approves Pfizer maternal RSV vaccine for infantsPfizer hopes that its respiratory syncytial virus vaccine will be available to the public by the end of October or the beginning of November.
Baca lebih lajut »
 Pfizer maternal RSV vaccine gets FDA approvalNew shot designed to protect babies against leading cause of hospitalization is expected to be available this fall.
Pfizer maternal RSV vaccine gets FDA approvalNew shot designed to protect babies against leading cause of hospitalization is expected to be available this fall.
Baca lebih lajut »
