Daily News | The FDA says a drug for preventing preterm births does not work. The drugmaker has pushed back, enlisting support of Black women.
A patient advocacy group met with a staff member for U.S. Rep. Madeleine Dean in July 2021, seeking her support for a drug that is designed to reduce the risk of preterm birth. The advocates said the drug, called Makena, was needed especially for Black women, who are at higher risk of delivering premature babies.
The FDA approved Makena in 2011 based on a study suggesting that it reduced the risk of preterm birth. But critics later identified statistical flaws in how that study was conducted.
“We never knew that the alliance was paid for by the manufacturer, and noticed a number of prominent women organizations on the board,” he said. “Rep. Dean has not, nor [has] anyone in our office, taken any action since the staffer met with the Preterm Birth Prevention Alliance.”Drugmakers have increasingly sought accelerated approval for their products since that pathway became an option in 1992, according to theat the Department of Health and Human Services.
He saw that women had been invited to join the trial if they were at risk of preterm birth, meaning they had delivered at least one baby prematurely in the past. But in the placebo group, the percentage of women who ended up having another preterm birth, 54.9%, wasTo Urato, that suggested something had gone wrong in how the women were chosen for the two groups. Upon looking at the details, he saw that prior to the trial, 41.
Indonesia Berita Terbaru, Indonesia Berita utama
Similar News:Anda juga dapat membaca berita serupa dengan ini yang kami kumpulkan dari sumber berita lain.
 Walmart is now selling over-the-counter hearing aids after FDA rule change | CNN BusinessWalmart announced Monday that its customers can for the first time buy over-the-counter hearing aids without a prescription and medical exam by a doctor.
Walmart is now selling over-the-counter hearing aids after FDA rule change | CNN BusinessWalmart announced Monday that its customers can for the first time buy over-the-counter hearing aids without a prescription and medical exam by a doctor.
Baca lebih lajut »
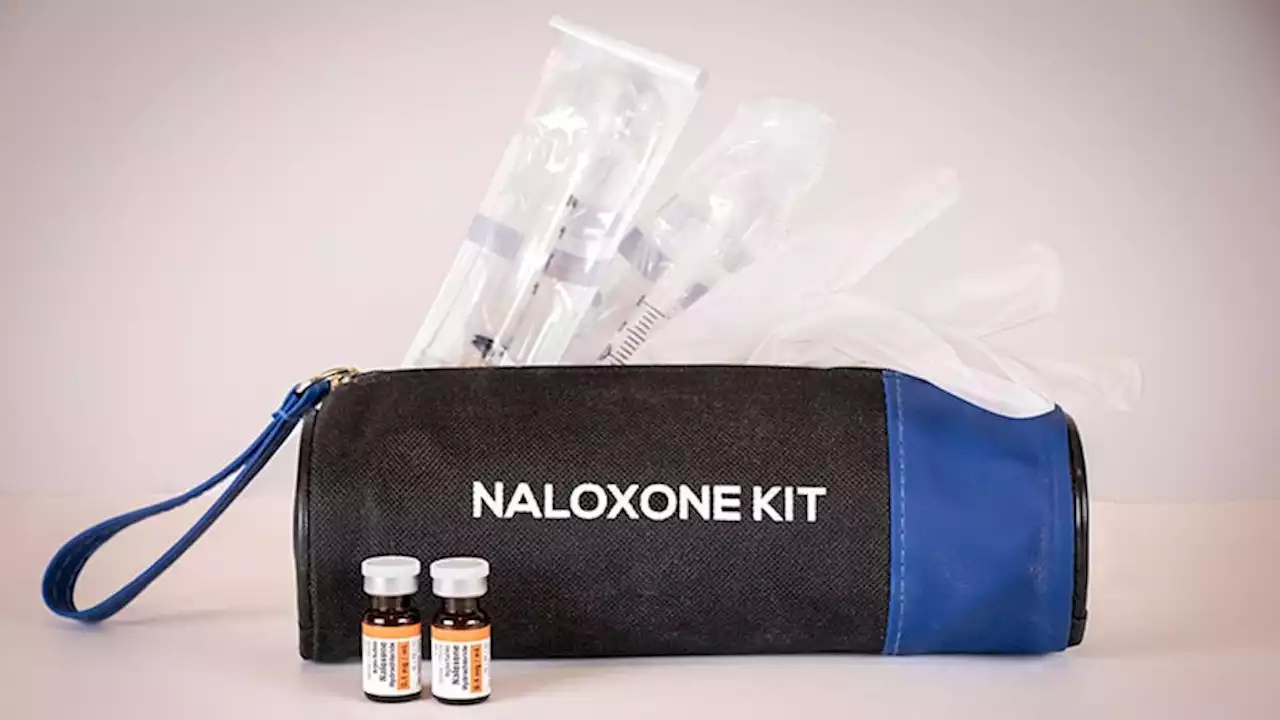 New FDA Guidance Aims to Boost Access to Opioid-Reversal DrugUnder new guidance, harm reduction programs distributing naloxone will be exempt from certain federal requirements in an effort to increase access to the opioid antidote.
New FDA Guidance Aims to Boost Access to Opioid-Reversal DrugUnder new guidance, harm reduction programs distributing naloxone will be exempt from certain federal requirements in an effort to increase access to the opioid antidote.
Baca lebih lajut »
 7 Cereals Can No Longer Claim ‘Healthy’ Label Under FDA RuleTo be considered “healthy,” cereals need three-fourth ounces of whole grains and no more than 1 gram of saturated fat, 230 milligrams of sodium, and 2.5 grams of added sugars. Seven common American brands that don’t meet the “healthy” label standards:
7 Cereals Can No Longer Claim ‘Healthy’ Label Under FDA RuleTo be considered “healthy,” cereals need three-fourth ounces of whole grains and no more than 1 gram of saturated fat, 230 milligrams of sodium, and 2.5 grams of added sugars. Seven common American brands that don’t meet the “healthy” label standards:
Baca lebih lajut »
