Check your freezer for these recalled products.
Shutterstock/progressman
According to the FDA, Amy's Kitchen decided to initiate their recall of its Vegan Organic Rice Mac & Cheeze when a third-party laboratory test discovered trace amounts of milk protein in one of the meals. The company says it is acting out of"an abundance of caution to ensure the safety of consumers who have an allergy or sensitivity to milk."
Recent legislation has changed the way food must be labeled for safety reasons. The Food Allergy Safety, Treatment, Education, and Research Act, requiring"Big 8" allergens such as milk to be on all packaged food regulated by the FDA, was signed into law on Apr. 23, 2021 and will go into effect on Jan. 1, 2023.Customers who purchased the affected products are advised to either throw them away or return them for a full refund. Anyone with questions or concerns about the recalled frozen chopped spinach can contact customer care for Lidl at 1-844-747-5435 Monday through Saturday from 8:00 a.m. to 8:00 p.m. EST.
Customers who may have purchased the recalled mac & cheese can contact Amy's Kitchen at 800-643-0570. Representatives are available on weekdays from 9 a.m. to 5 p.m. PST.Zach is a freelance writer specializing in beer, wine, food, spirits, and travel. He is based in Manhattan.
Indonesia Berita Terbaru, Indonesia Berita utama
Similar News:Anda juga dapat membaca berita serupa dengan ini yang kami kumpulkan dari sumber berita lain.
 FDA Curbs Use of COVID-19 Antibody Drugs Sidelined by OmicronU.S. health officials say COVID-19 antibody drugs from Regeneron and Eli Lilly should no longer be used because they are unlikely to work against the omicron variant.
FDA Curbs Use of COVID-19 Antibody Drugs Sidelined by OmicronU.S. health officials say COVID-19 antibody drugs from Regeneron and Eli Lilly should no longer be used because they are unlikely to work against the omicron variant.
Baca lebih lajut »
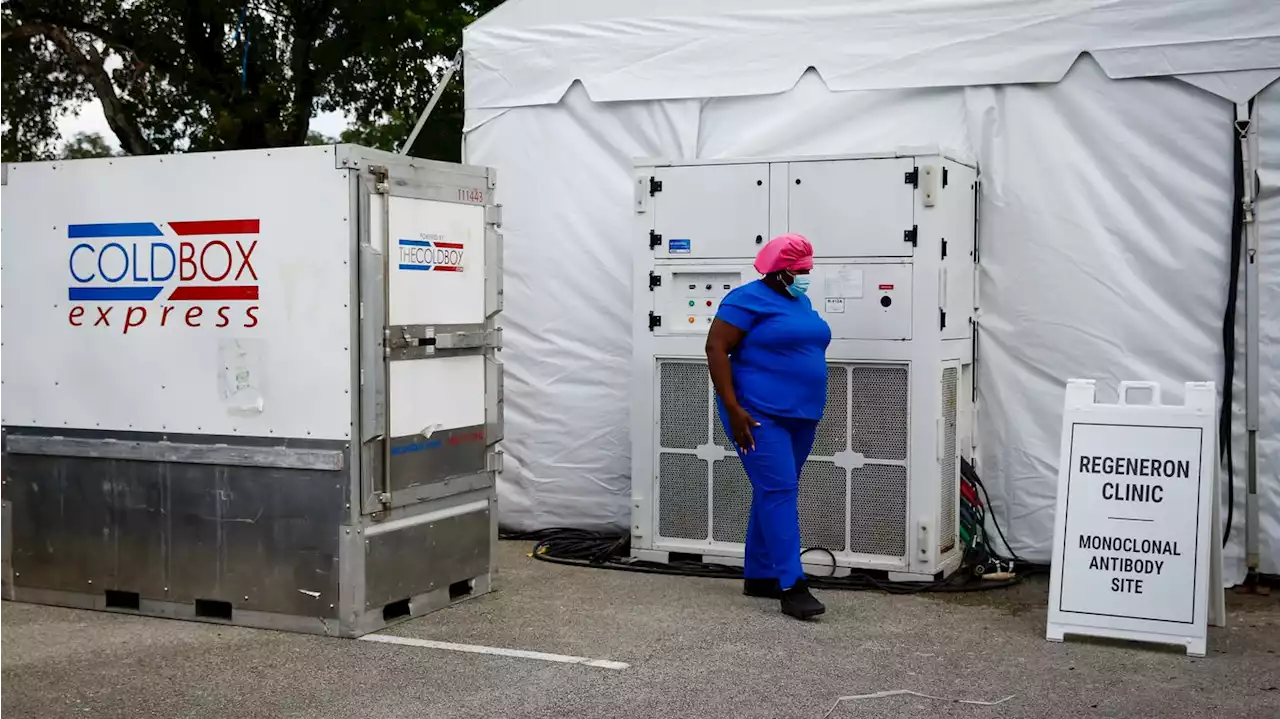 FDA limits use of Regeneron and Lilly COVID antibody treatmentsThe FDA said 'data show these treatments are highly unlikely to be active' against Omicron.
FDA limits use of Regeneron and Lilly COVID antibody treatmentsThe FDA said 'data show these treatments are highly unlikely to be active' against Omicron.
Baca lebih lajut »
 FDA halts use of antibody drugs that don’t work against omicronCOVID-19 antibody drugs from Regeneron and Eli Lilly should no longer be used because they don’t work against the omicron variant that now accounts for nearly all U.S. infections, U.S. health regulators said Monday.
FDA halts use of antibody drugs that don’t work against omicronCOVID-19 antibody drugs from Regeneron and Eli Lilly should no longer be used because they don’t work against the omicron variant that now accounts for nearly all U.S. infections, U.S. health regulators said Monday.
Baca lebih lajut »
 FDA halts use of monoclonal antibody drugs from Regeneron, Eli Lilly that don't work vs. omicronCOVID-19 antibody drugs from Regeneron and Eli Lilly should no longer be used because they don't work against the omicron variant, U.S. health officials said.
FDA halts use of monoclonal antibody drugs from Regeneron, Eli Lilly that don't work vs. omicronCOVID-19 antibody drugs from Regeneron and Eli Lilly should no longer be used because they don't work against the omicron variant, U.S. health officials said.
Baca lebih lajut »
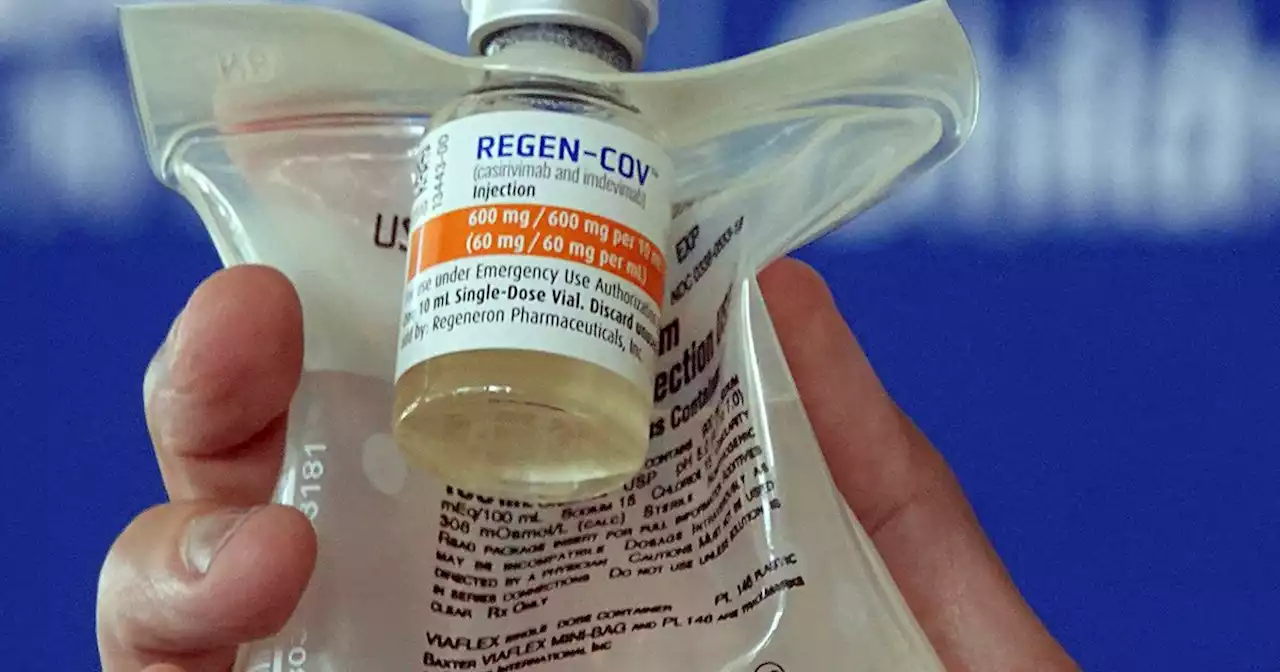 FDA halts use of antibody drugs that don’t work vs. omicronWASHINGTON (AP) — COVID-19 antibody drugs from Regeneron and Eli Lilly should no longer be used because they don’t work against the omicron variant that...
FDA halts use of antibody drugs that don’t work vs. omicronWASHINGTON (AP) — COVID-19 antibody drugs from Regeneron and Eli Lilly should no longer be used because they don’t work against the omicron variant that...
Baca lebih lajut »
 FDA halts use of antibody drugs that don't work against omicronCOVID-19 antibody drugs from Regeneron and Eli Lilly should no longer be used because they don’t work against the omicron variant. Doctors have alternate therapies, including two new antiviral pills from Pfizer and Merck, but both are in short supply.
FDA halts use of antibody drugs that don't work against omicronCOVID-19 antibody drugs from Regeneron and Eli Lilly should no longer be used because they don’t work against the omicron variant. Doctors have alternate therapies, including two new antiviral pills from Pfizer and Merck, but both are in short supply.
Baca lebih lajut »
