A first-in-human study of an antisense oligonucleotide targeting hepatitis B virus RNA provides initial insights into this potential new therapeutic modality for individuals with chronic HBV infection ASO HKUniversity NMEDClinical WorldHepatitisDay
. Twice weekly dosing in weeks 1 and 2 constituted loading doses, with the aim of reaching steady-state hepatic concentrations following the week 3 dose instead of 13–15 weeks without loading. The main assessments of single-agent treatment effects of bepirovirsen were performed on day 29, 7 d after the last dose of study drug . After these assessments, all patients received daily treatment with NA to end of study . Patients already on a stable NA regimen received treatment throughout .
Secondary objectives and endpoints were to: examine the effects of bepirovirsen administration on plasma HBV DNA concentration ; examine the effects of bepirovirsen administration on serum HBsAg concentration ; examine the effect of bepirovirsen administration on serum HBeAg concentration in patients who were HBeAg positive at baseline ; assess plasma pharmacokinetics of bepirovirsen in patients with chronic HBV infection ; and describe the safety and tolerability of tenofovir disoproxil...
Exploratory endpoints and objectives included describing the rate of seroconversion to anti-HBs or anti-HBe antibody-positive during treatment with bepirovirsen and then during subsequent treatment with TDF, or ETV if administered .Inclusion criteria were chronic HBV infection ≥6 months and serum HBsAg ≥50 IU ml; both HBeAg-positive and HBeAg-negative patients could participate. Treatment-naïve patients had a plasma HBV DNA ≥2 × 10.
Although the Data and Safety Monitoring Board supported dose escalation to 450 mg, it was acknowledged that further evaluation of the 300 mg dose was warranted and the sponsor continued evaluation of the 300 mg dose in the third cohort to allow further characterization of the antiviral effect of bepirovirsen at the 300 mg dose level. One exploratory cohort was added to evaluate add-on treatment with bepirovirsen 300 mg versus placebo in on-NA patients with CHB.
All participants, study monitors, study center personnel and contract research organization personnel were blinded to treatment assignment. A global protocol amendment was made to include an on-NA cohort, in which participants with CHB already on a stable regimen of TDF or ETV were treated with 300 mg of bepirovirsen by the same dosing schedule as treatment-naïve patients; all applicable sections of the protocol were updated to reflect the addition of the on-NA cohort.
Indonesia Berita Terbaru, Indonesia Berita utama
Similar News:Anda juga dapat membaca berita serupa dengan ini yang kami kumpulkan dari sumber berita lain.
 Financing the health needs of Ukrainian refugees - Nature MedicineFinancing the health needs of Ukrainian refugees. Correspondence from Wu Zeng Georgetown.
Financing the health needs of Ukrainian refugees - Nature MedicineFinancing the health needs of Ukrainian refugees. Correspondence from Wu Zeng Georgetown.
Baca lebih lajut »
 Antivirals May Reduce Hospitalizations and Deaths in COVIDPatients with nonsevere COVID may avoid progression to hospitalization or death through treatment with nirmatrelvir-ritonavir or molnupiravir, according to a systematic review and meta-analysis.
Antivirals May Reduce Hospitalizations and Deaths in COVIDPatients with nonsevere COVID may avoid progression to hospitalization or death through treatment with nirmatrelvir-ritonavir or molnupiravir, according to a systematic review and meta-analysis.
Baca lebih lajut »
 Symptoms and risk factors for long COVID in non-hospitalized adults - Nature MedicineA retrospective analysis of primary care records in the UK reveals individual symptoms associated with SARS-CoV-2 infections that persisted for 12 weeks or more post-infection, as well as risk factors associated with developing LongCOVID unibirmingham
Symptoms and risk factors for long COVID in non-hospitalized adults - Nature MedicineA retrospective analysis of primary care records in the UK reveals individual symptoms associated with SARS-CoV-2 infections that persisted for 12 weeks or more post-infection, as well as risk factors associated with developing LongCOVID unibirmingham
Baca lebih lajut »
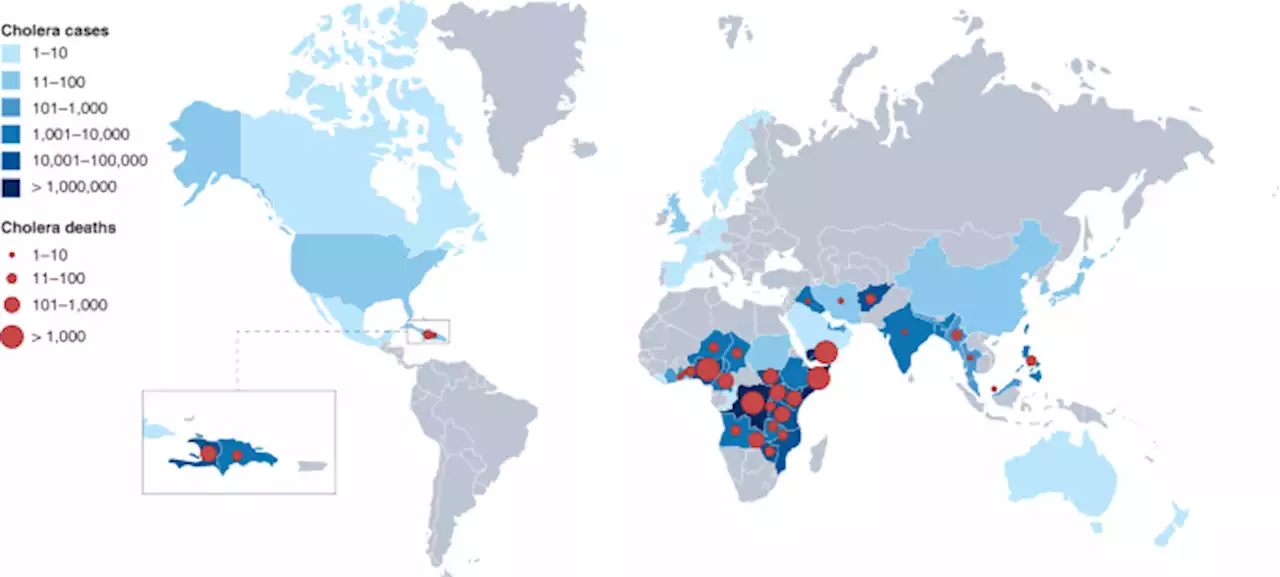 A blueprint for eliminating cholera by 2030 - Nature MedicineCholera is endemic in 47 countries, but deaths from this disease can be eliminated with a package of low-cost measures implemented by community healthcare workers.
A blueprint for eliminating cholera by 2030 - Nature MedicineCholera is endemic in 47 countries, but deaths from this disease can be eliminated with a package of low-cost measures implemented by community healthcare workers.
Baca lebih lajut »
 Astros drop second in a row at OaklandChad Pinder hits grand slam off Luis Garcia to give A's the victory
Astros drop second in a row at OaklandChad Pinder hits grand slam off Luis Garcia to give A's the victory
Baca lebih lajut »
