Pfizer said it expects to price its Covid-19 vaccine at $110 to $130 a dose for adults when the U.S. government stops purchases
The drugmaker laid out a potential price as it prepares to sell the shot commercially, perhaps early next yearTony Luong for The Wall Street JournalPfizer Inc. gave some details about its plans to sell the Covid-19 vaccine it developed with BioNTech SE on the commercial market in the U.S., saying it expects to price the shot at $110 to $130 per dose for adults.
The drugmaker is still negotiating with health insurers, but anticipates the discussions will lead to a list price in the range, Angela Lukin, a Pfizer official, said Thursday.
Indonesia Berita Terbaru, Indonesia Berita utama
Similar News:Anda juga dapat membaca berita serupa dengan ini yang kami kumpulkan dari sumber berita lain.
 Cancer vaccine could be available by 2030, COVID vax-makers sayA vaccine for cancer may be a reality within the decade, according to a husband and wife duo behind the successful Pfizer/BioNtech COVID-19 vaccine.
Cancer vaccine could be available by 2030, COVID vax-makers sayA vaccine for cancer may be a reality within the decade, according to a husband and wife duo behind the successful Pfizer/BioNtech COVID-19 vaccine.
Baca lebih lajut »
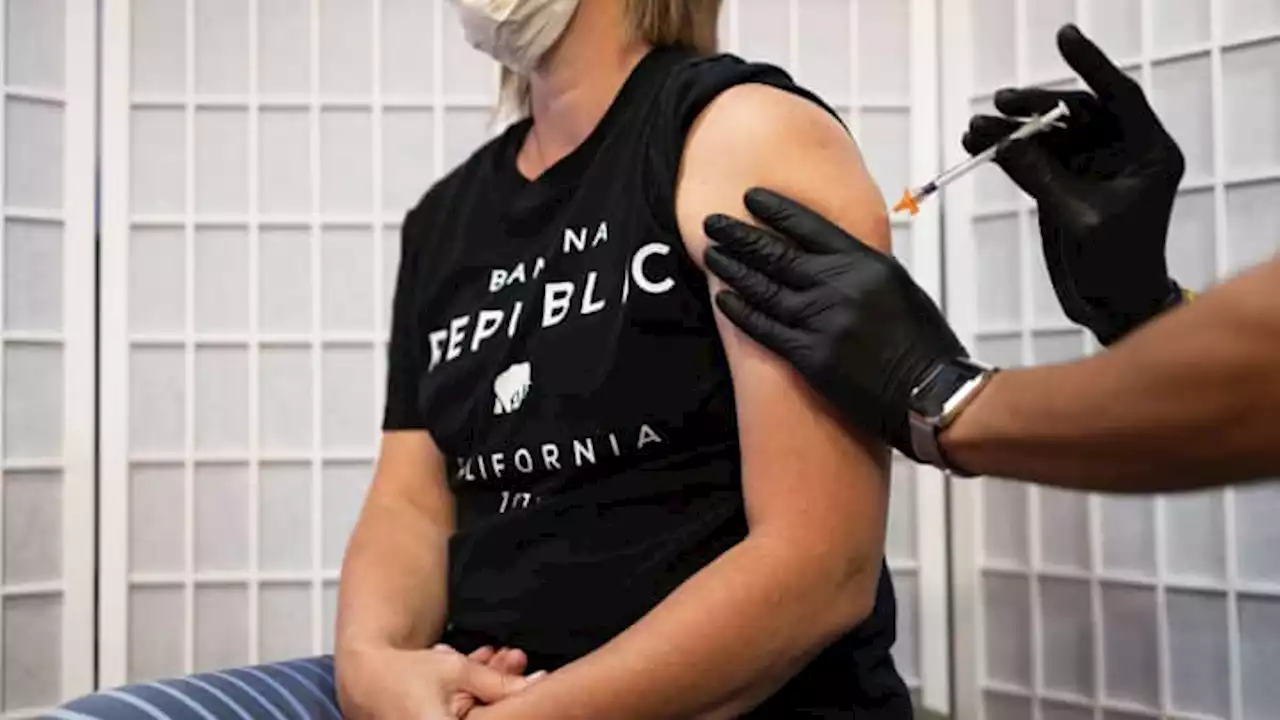 FDA authorizes Novavax's Covid shots as mix-and-match booster to Pfizer or ModernaNovavax's Covid shots rely on protein technology used in other vaccines for decades, rather than the messenger RNA used in the Pfizer and Moderna vaccines.
FDA authorizes Novavax's Covid shots as mix-and-match booster to Pfizer or ModernaNovavax's Covid shots rely on protein technology used in other vaccines for decades, rather than the messenger RNA used in the Pfizer and Moderna vaccines.
Baca lebih lajut »
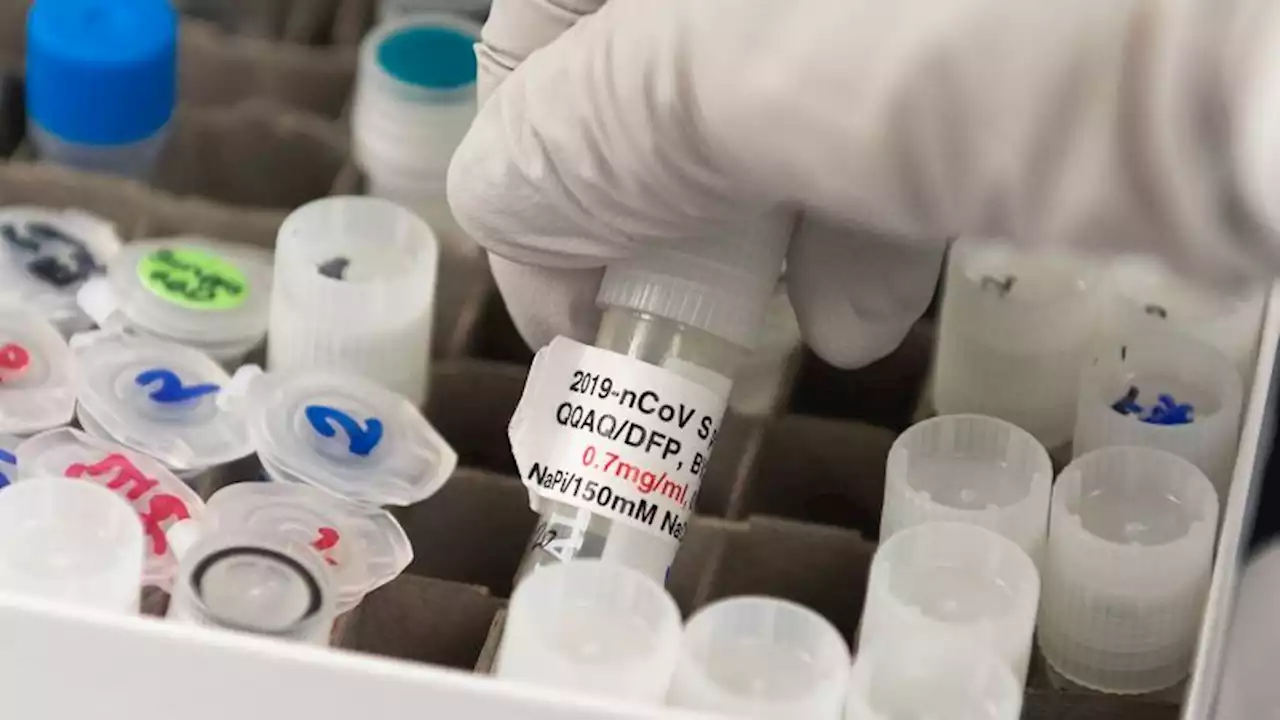 FDA, CDC sign off on Novavax Covid-19 vaccine as a first booster shot | CNNThe US Food and Drug Administration authorized Novavax’s Covid-19 vaccine for use as a first booster dose among adults 18 and older, at least six months after completing a primary Covid-19 vaccine series.
FDA, CDC sign off on Novavax Covid-19 vaccine as a first booster shot | CNNThe US Food and Drug Administration authorized Novavax’s Covid-19 vaccine for use as a first booster dose among adults 18 and older, at least six months after completing a primary Covid-19 vaccine series.
Baca lebih lajut »
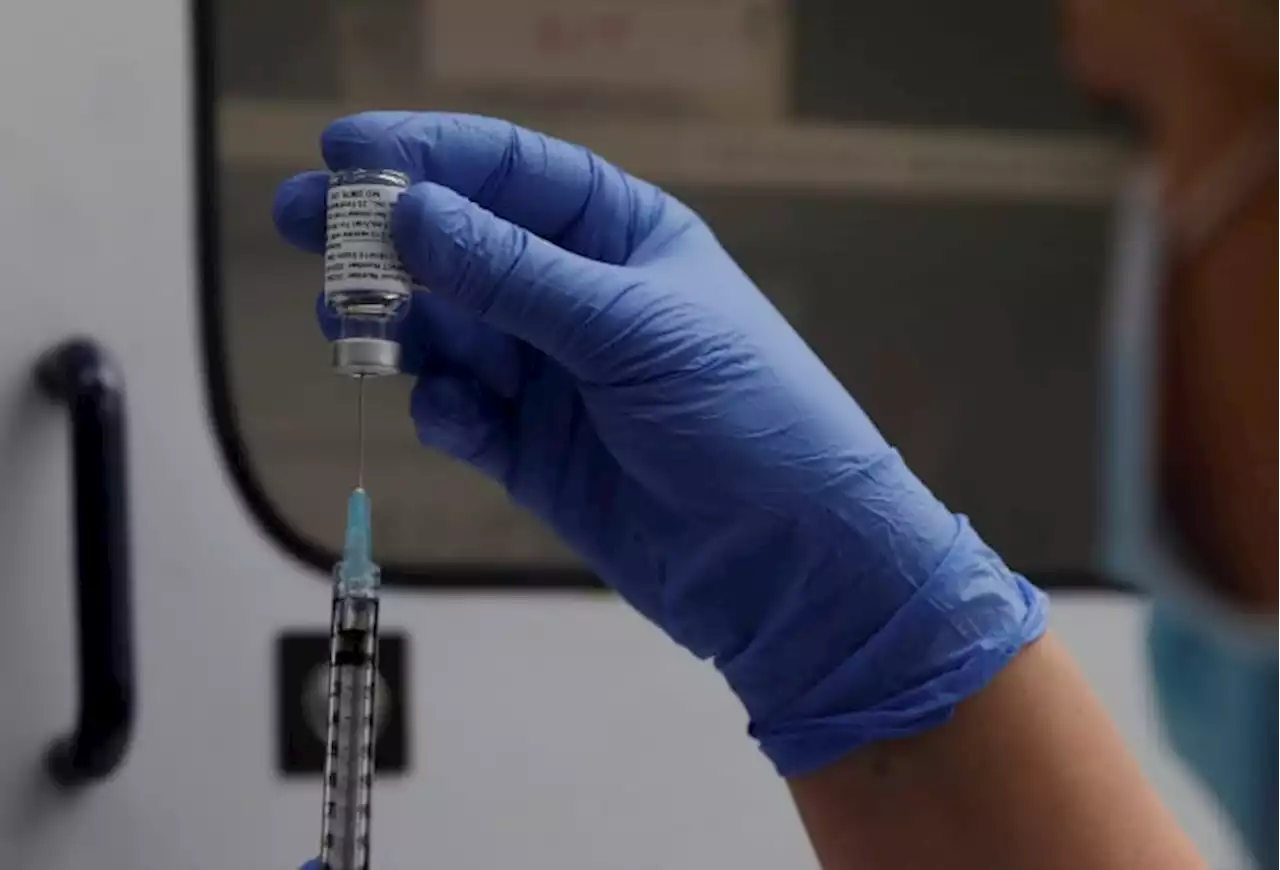 US clears Novavax COVID booster doseThe Food and Drug Administration has authorized a booster dose of the COVID-19 vaccine made by Novavax.
US clears Novavax COVID booster doseThe Food and Drug Administration has authorized a booster dose of the COVID-19 vaccine made by Novavax.
Baca lebih lajut »
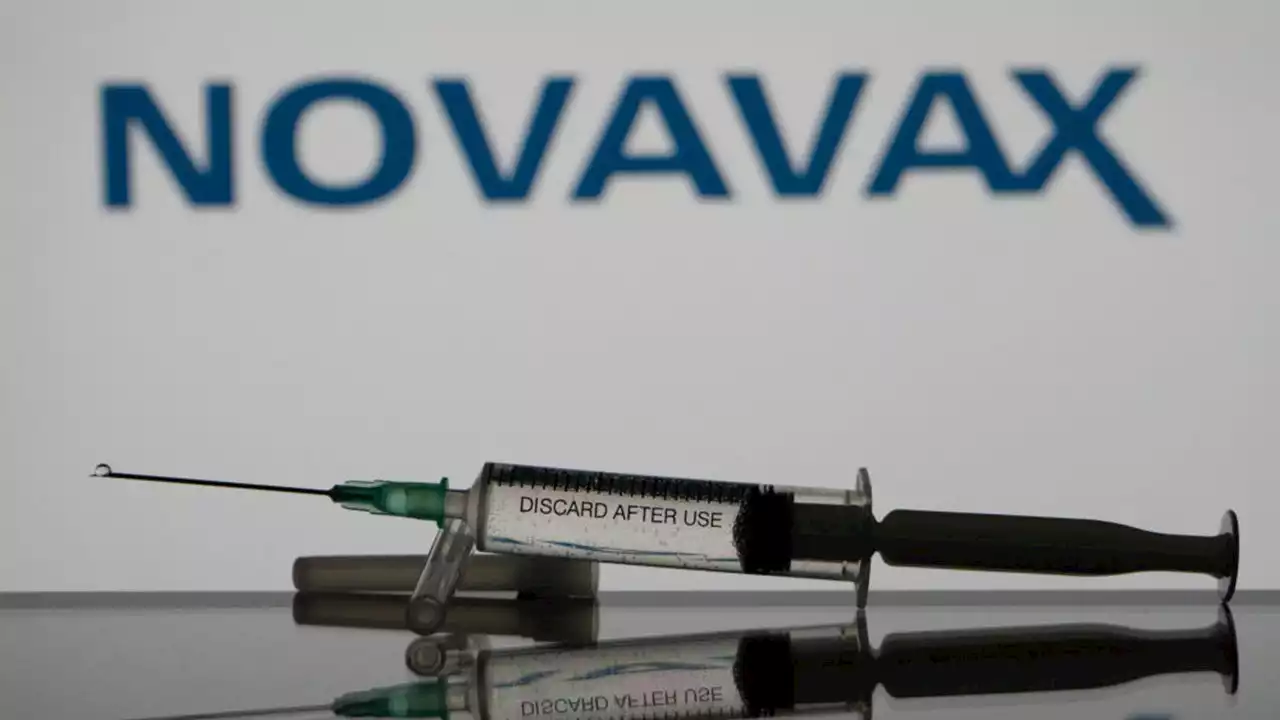 FDA OKs Novavax COVID-19 booster doseThe Food and Drug Administration has authorized a booster dose of the COVID-19 vaccine made by Novavax.
FDA OKs Novavax COVID-19 booster doseThe Food and Drug Administration has authorized a booster dose of the COVID-19 vaccine made by Novavax.
Baca lebih lajut »
