A third pediatric dose of the Pfizer-BioNTech COVID-19 vaccine in children 6 months to under 5 years of age prompted a strong immune response, with a safety profile that was similar to placebo, the companies said:
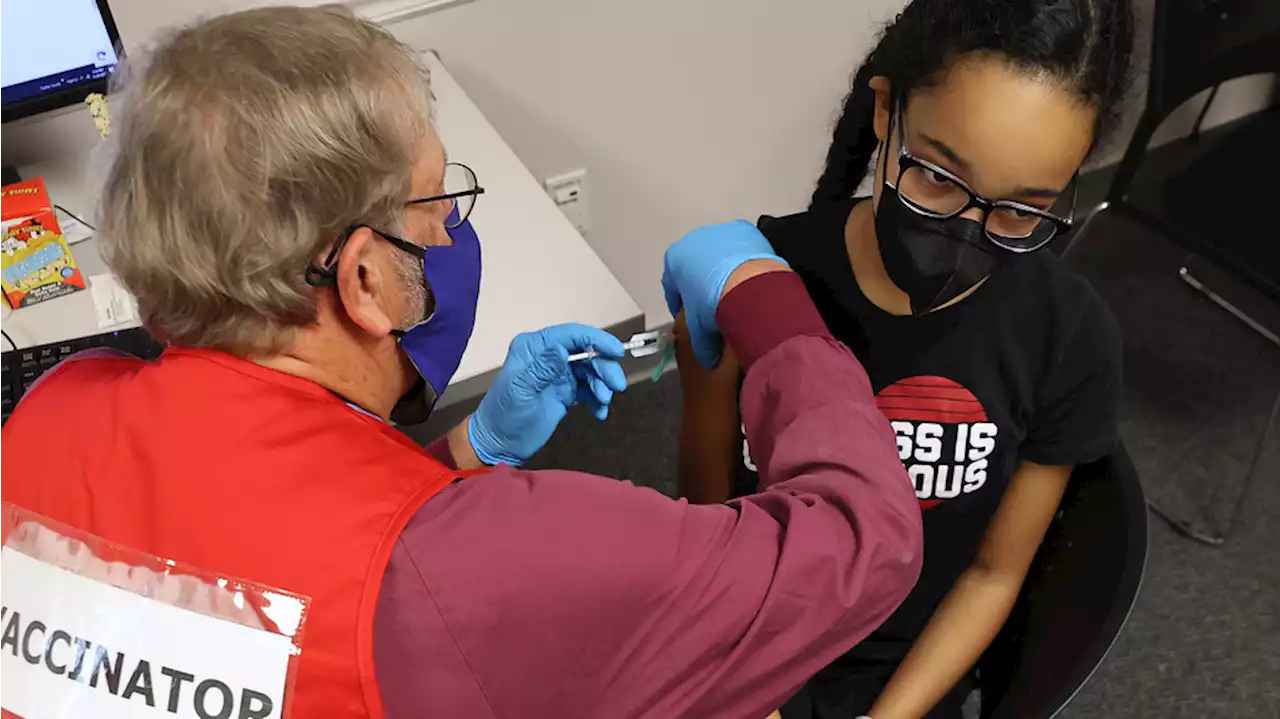
Pfizer will submit new data to the FDA this week about trials of its vaccine for kids younger than 5 years old. Here, an older child receives the Pfizer BioNTech COVID-19 vaccine in Virginia last November.
Pfizer's pediatric COVID-19 vaccine has an efficacy of 80.3%, according to a preliminary analysis, and meets"all immunobridging criteria required for Emergency Use Authorization," the company said Monday. The results are based on clinical trials in which kids from six months to age 5 got three doses of the company's vaccine.
Berita ini telah kami rangkum agar Anda dapat membacanya dengan cepat. Jika Anda tertarik dengan beritanya, Anda dapat membaca teks lengkapnya di sini. Baca lebih lajut: