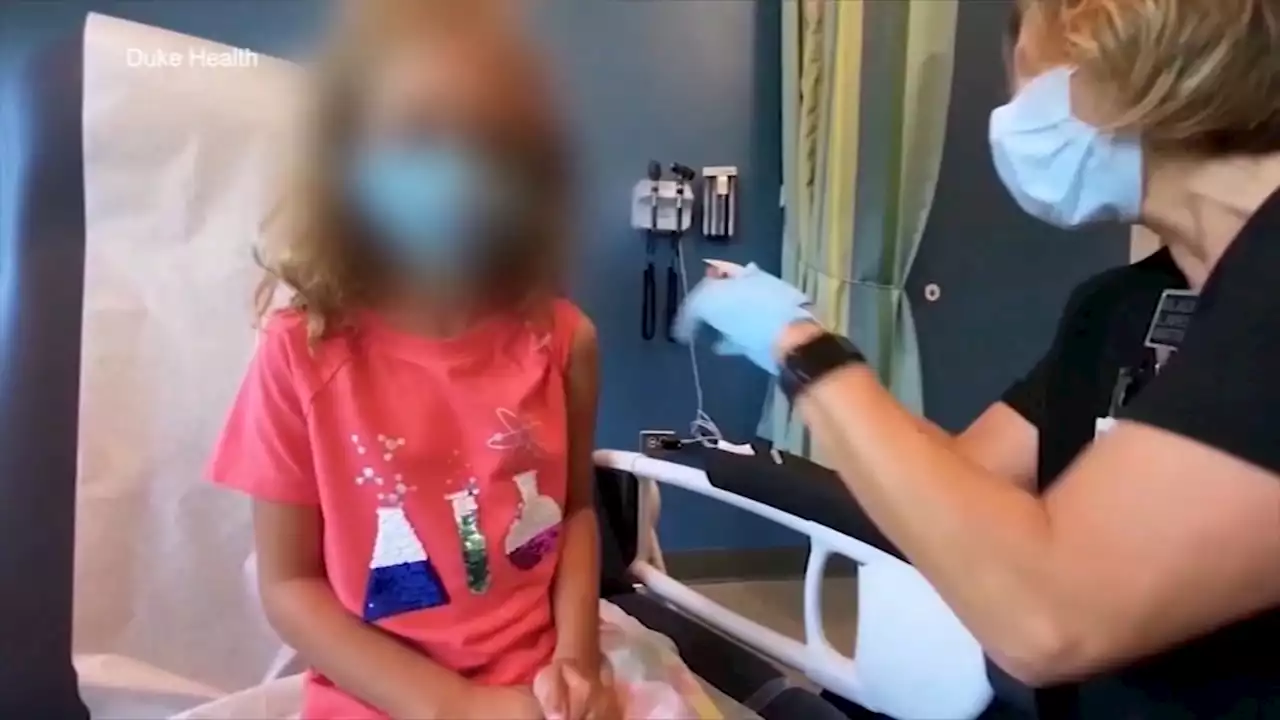
Children as young as 6 months old could be eligible for the COVID-19 vaccine later this month.
U.S. regulators are urging drugmaker Pfizer to apply for emergency authorization for a two-dose regimen of its COVID-19 vaccine for children 6 months to 5 years old while awaiting data on a three-dose course, aiming to clear the way for the shots as soon as late February, a person familiar with the matter told The Associated Press Monday.
The person spoke on the condition of anonymity to discuss sensitive regulatory issues. The person said the decreased effectiveness of the two-dose vaccine was not unexpected given the emergence of the highly transmissible omicron variant of COVID-19. Allowing young kids to be vaccinated with a two-dose shot earlier would ultimately accelerate when they could get the expected stronger protection from a third dose.
Young children are far less likely than adults to develop serious complications or to die from COVID-19, but incidences of illness among the age group have risen amid the nationwide spike in cases from the omicron variant. Most cases and deaths occur among older people, especially those who are unvaccinated.
Indonesia Berita Terbaru, Indonesia Berita utama
Similar News:Anda juga dapat membaca berita serupa dengan ini yang kami kumpulkan dari sumber berita lain.
 US Urges Pfizer to Apply for Under-5 COVID Shots: AP SourceU.S. regulators are urging drugmaker Pfizer to apply for emergency authorization for a two-dose regimen of its COVID-19 vaccine for children 6 months to 5 years old
US Urges Pfizer to Apply for Under-5 COVID Shots: AP SourceU.S. regulators are urging drugmaker Pfizer to apply for emergency authorization for a two-dose regimen of its COVID-19 vaccine for children 6 months to 5 years old
Baca lebih lajut »
 AP source: US urges Pfizer to apply for under-5 COVID shotsRegulators are urging Pfizer to apply for emergency authorization for a two-dose regimen of its COVID-19 vaccine for children 6 months-5 years old while awaiting data on a three-dose course, aiming to clear the way for the shots as soon as late February.
AP source: US urges Pfizer to apply for under-5 COVID shotsRegulators are urging Pfizer to apply for emergency authorization for a two-dose regimen of its COVID-19 vaccine for children 6 months-5 years old while awaiting data on a three-dose course, aiming to clear the way for the shots as soon as late February.
Baca lebih lajut »
 GOP-Led States Take Aim at Covid-19 Vaccine Mandates for School ChildrenFourteen states have measures pending to ban or limit Covid-19 vaccine mandates for school children, and recent polling finds a majority of U.S. parents opposed to mandates
GOP-Led States Take Aim at Covid-19 Vaccine Mandates for School ChildrenFourteen states have measures pending to ban or limit Covid-19 vaccine mandates for school children, and recent polling finds a majority of U.S. parents opposed to mandates
Baca lebih lajut »
 Vaccinated Parents Help Protect Children from COVID: StudyParents who keep up to date with their COVID-19 vaccines may help protect their unvaccinated children from the disease in the process, new research shows.
Vaccinated Parents Help Protect Children from COVID: StudyParents who keep up to date with their COVID-19 vaccines may help protect their unvaccinated children from the disease in the process, new research shows.
Baca lebih lajut »
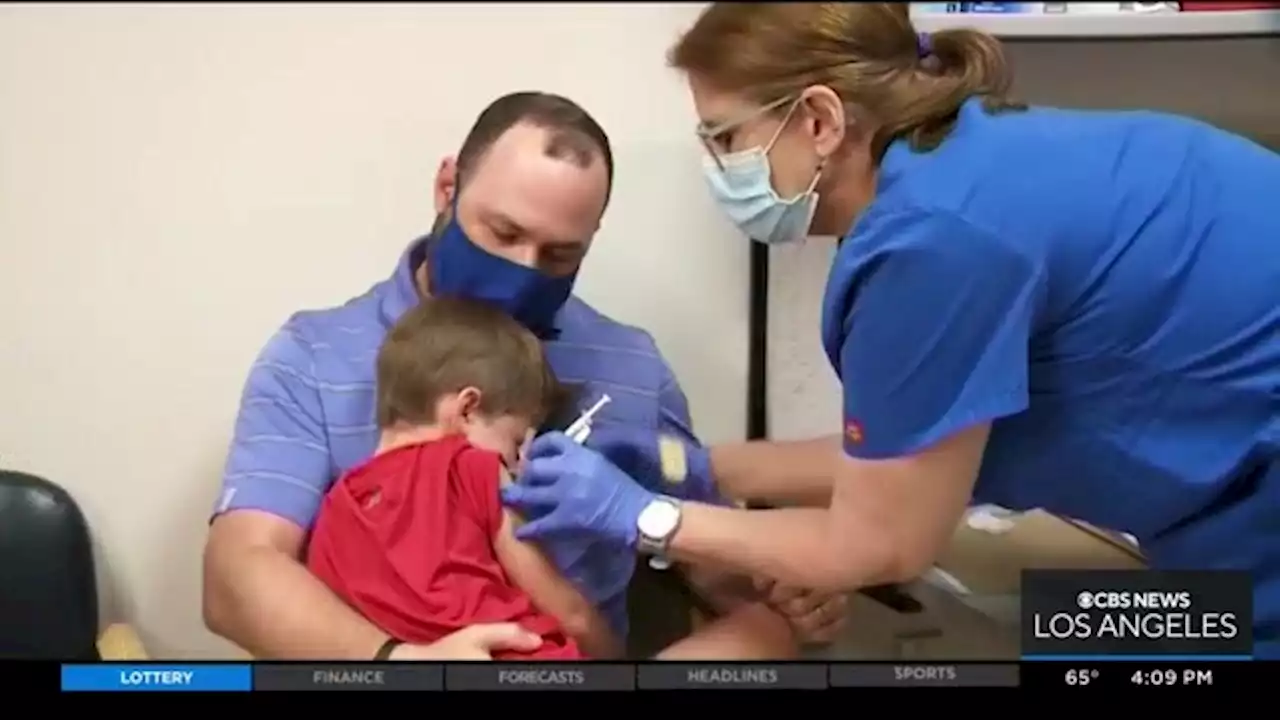 Children Under 5-Years-Old May Soon Be Eligible For COVID VaccineClinical trials on little kids are underway on a two-dose vaccine and a three-dose shot. The goal is to prevent severe disease.
Children Under 5-Years-Old May Soon Be Eligible For COVID VaccineClinical trials on little kids are underway on a two-dose vaccine and a three-dose shot. The goal is to prevent severe disease.
Baca lebih lajut »
 Pfizer vaccine for children under 5 may be available in U.S. by end-Feb - reportPfizer Inc and Germany's BioNTech SE are expected to submit an emergency use authorization request as early as Tuesday to the U.S. Food and Drug Administration (FDA) for vaccines for children aged six months to 5 years, the Washington Post reported on Monday.
Pfizer vaccine for children under 5 may be available in U.S. by end-Feb - reportPfizer Inc and Germany's BioNTech SE are expected to submit an emergency use authorization request as early as Tuesday to the U.S. Food and Drug Administration (FDA) for vaccines for children aged six months to 5 years, the Washington Post reported on Monday.
Baca lebih lajut »