The Food and Drug Administration’s independent experts will review the safety and effectiveness of Novavax’s Covid vaccine Tuesday. If they endorse the vaccine, the FDA will almost certainly authorize the shot for adults in the U.S. Novavax’s vaccine uses protein technology that is different Pfizer and Moderna’s messenger RNA shots. Novavax was part of the U.S. race to develop a…
Bill Gates Has 5 Book Recommendations for Your 2022 Summer Reading List: ‘Compelling Without Sacrificing Any Complexity'Pfizer and Moderna's shots rely on messenger RNA to turn human cells into factories that produce copies of Covid's spike protein to induce an immune response that fights the virus. The spike is the part of the virus that latches onto and invades human cells.
"We know exactly what we've made and we test it as part of the process of releasing the vaccine to assure that it's in the right conformation," Dubovsky said. Novavax's adult trial was conducted from December 2020 through September of 2021, before the omicron variant became dominant. There is no data available to assess Novavax's effectiveness against omicron, which continues to mutate into more transmissible versions of the virus, according to FDA briefing documents published ahead of Tuesday's committee meeting. However, FDA officials said the two-dose vaccine would more likely than provide meaningful protection against severe disease.
To be clear, the FDA committee is only reviewing Novavax's two-dose primary series for adults on Tuesday. However, Novavax plans to ask the FDA to authorize a third dose for adults as well as the primary series for teenagers 12 to 17 if the agency clears two doses for adults, Taylor said. Novavax is also studying a third shot for teenagers.The most common side effects of Novavax's shots were injection site pain, fatigue, headache and muscle pain, according to FDA briefing documents.
Trizzino said an initial delivery of shots will be made in the weeks after FDA authorization without specifying an amount. Novavax and the U.S. government have not reached an agreement yet on future orders, he said.
Indonesia Berita Terbaru, Indonesia Berita utama
Similar News:Anda juga dapat membaca berita serupa dengan ini yang kami kumpulkan dari sumber berita lain.
 FDA Advisers Consider Novavax COVID-19 Vaccine This WeekThe FDA’s vaccine advisors are scheduled to meet on Tuesday to consider authorizing the Novavax COVID-19 vaccine. The committee will vote on whether the benefits of the two-dose vaccine outweigh the risks for ages 18 and older.
FDA Advisers Consider Novavax COVID-19 Vaccine This WeekThe FDA’s vaccine advisors are scheduled to meet on Tuesday to consider authorizing the Novavax COVID-19 vaccine. The committee will vote on whether the benefits of the two-dose vaccine outweigh the risks for ages 18 and older.
Baca lebih lajut »
 Hepatitis A Outbreak Likely Caused by Strawberries, FDA SaysSo far, 17 cases have been identified in the U.S., with 15 in California and one each in Minnesota and North Dakota. Canada has identified 10 cases, with six in Saskatchewan and four in Alberta.
Hepatitis A Outbreak Likely Caused by Strawberries, FDA SaysSo far, 17 cases have been identified in the U.S., with 15 in California and one each in Minnesota and North Dakota. Canada has identified 10 cases, with six in Saskatchewan and four in Alberta.
Baca lebih lajut »
 FDA Advisers Consider Novavax COVID-19 Vaccine This WeekThe FDA’s vaccine advisors are scheduled to meet on Tuesday to consider authorizing the Novavax COVID-19 vaccine. The committee will vote on whether the benefits of the two-dose vaccine outweigh the risks for ages 18 and older.
FDA Advisers Consider Novavax COVID-19 Vaccine This WeekThe FDA’s vaccine advisors are scheduled to meet on Tuesday to consider authorizing the Novavax COVID-19 vaccine. The committee will vote on whether the benefits of the two-dose vaccine outweigh the risks for ages 18 and older.
Baca lebih lajut »
 A more traditional coronavirus shot is on the way. Some people can’t wait.More than a year after people began rolling up their sleeves for cutting-edge coronavirus shots, a new vaccine — this one based on a classic, decades-old technology — is expected to begin rolling out in the United States this summer.
A more traditional coronavirus shot is on the way. Some people can’t wait.More than a year after people began rolling up their sleeves for cutting-edge coronavirus shots, a new vaccine — this one based on a classic, decades-old technology — is expected to begin rolling out in the United States this summer.
Baca lebih lajut »
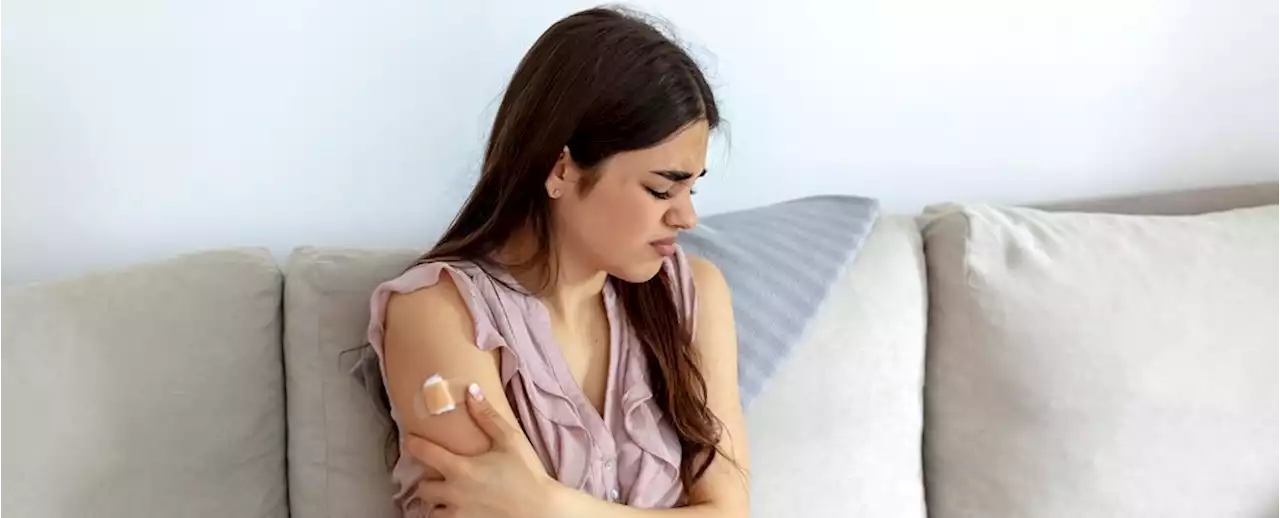 A Tiny Change in Jab Strategy Might Reduce COVID-19 Vaccine Fatigue, Mouse Study FindsFirst there's weakness and throbbing in the arm muscles that take on the immune-teaching chemicals. Then the fatigue and aches spread until your whole body is complaining.
A Tiny Change in Jab Strategy Might Reduce COVID-19 Vaccine Fatigue, Mouse Study FindsFirst there's weakness and throbbing in the arm muscles that take on the immune-teaching chemicals. Then the fatigue and aches spread until your whole body is complaining.
Baca lebih lajut »
