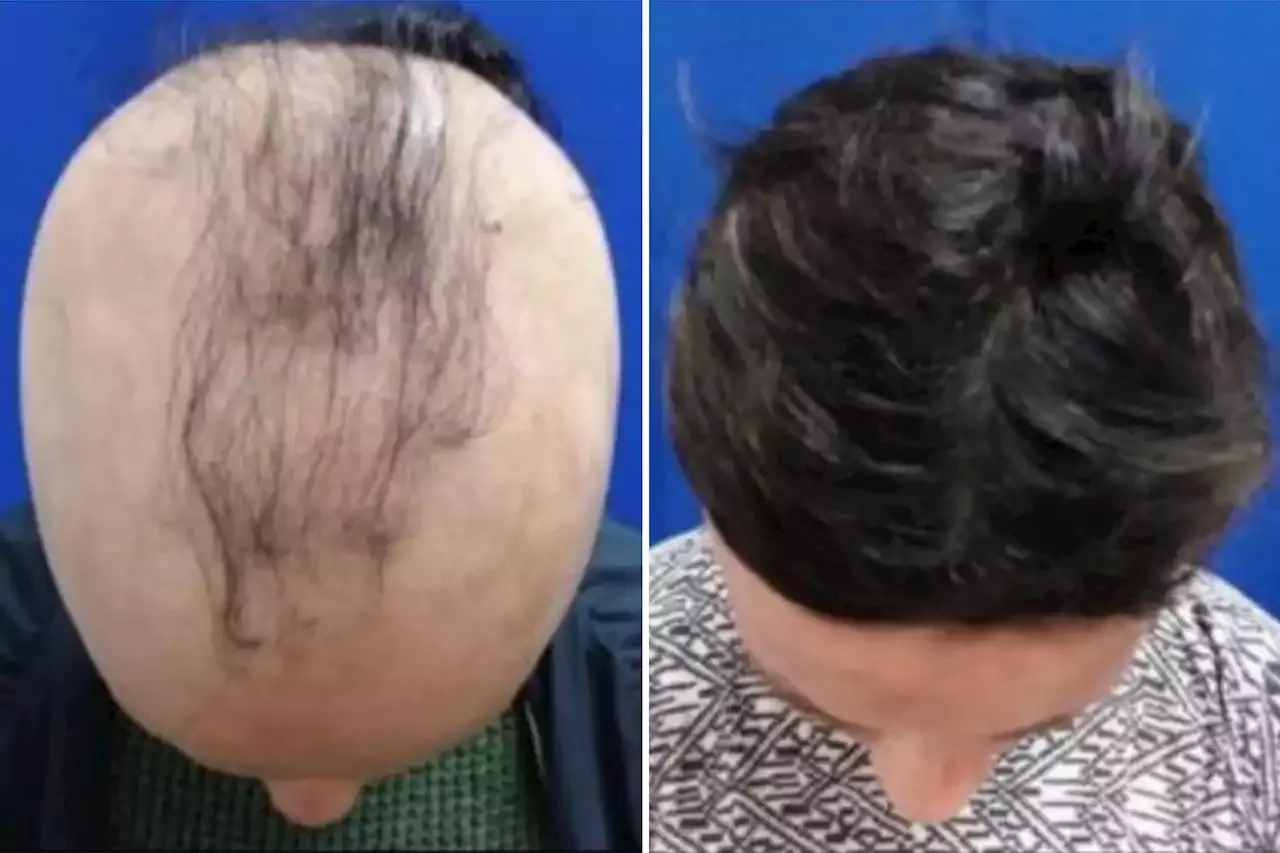
The latest trial studied 706 adults who have alopecia, aged 18 to 65, for 24 weeks in the US, Canada and Europe.
On average, the patients studied only had 16 percent of their hair at the start of the trial, with no one having more than 50 percent.
They were split into three groups: one was given a placebo, another an 8 milligrams twice-daily dose, and lastly a 12-milligram, twice-daily pill. Both groups taking the non-placebo doses saw regrowth, with a total of 41.5 percent of the stronger dose recipients experiencing 80 percent of hair regrowth. Among those that received the lower dose, nearly 30 percent experienced the same amount of hair regrowth. In the placebo group, only 0.8 percent of the participants saw more than 80% of hair growth.
After repeating the phase three trial again on 517 more patients, the company wants to present its findings and apply for US Food and Drug Administration approval next year. “We are extremely grateful to the patients and teams of clinical research professionals who participate in our trials,” said King in the statement. “We’re working to change the treatment landscape and hope that CTP-543 will be one of the first FDA-approved treatment options for this serious disease.”