Moderna's Chief Medical Officer talks one-on-one with ABC15 about COVID booster vaccines abc15
PHOENIX — A handful of new Arizona laws are in effect regarding COVID-19, from banning forced business closures to outlawing mask and vaccine mandates. Still, doctors and the CDC insist getting vaccinated and boosted is your best protection against hospitalization and COVID long-haul symptoms.
In an ABC15 exclusive one-on-one interview with Moderna Chief Medical Officer Dr. Paul Burton, he explained the problems have been high demand and delivery issues. He says Moderna's latest vaccine adds in what they call a messenger RNA which specifically targets the newest variant. As we are seeing new, more potent mutations, he also responded to President Biden's recent comments about the pandemic being over.
Indonesia Berita Terbaru, Indonesia Berita utama
Similar News:Anda juga dapat membaca berita serupa dengan ini yang kami kumpulkan dari sumber berita lain.
 Pfizer Asks FDA to Authorize Omicron Covid Booster Shots for Kids Ages 5 to 11Pfizer’s application to the FDA comes before clinical trial results of the omicron BA.5 booster shots have been published.
Pfizer Asks FDA to Authorize Omicron Covid Booster Shots for Kids Ages 5 to 11Pfizer’s application to the FDA comes before clinical trial results of the omicron BA.5 booster shots have been published.
Baca lebih lajut »
 Pfizer-BioNTech asks FDA to authorize new omicron-targeting COVID booster for children 5 to 11Pfizer-BioNTech submitted an application for the FDA to authorize its BA.4/BA.5 bivalent COVID-19 vaccine for kids 5 to 11 at a 10-microgram dose.
Pfizer-BioNTech asks FDA to authorize new omicron-targeting COVID booster for children 5 to 11Pfizer-BioNTech submitted an application for the FDA to authorize its BA.4/BA.5 bivalent COVID-19 vaccine for kids 5 to 11 at a 10-microgram dose.
Baca lebih lajut »
 Pfizer seeks to expand COVID omicron booster to 5- to 11-year-oldsIf the FDA agrees, children would start getting a kid-sized dose of the new omicron-targeted formula.
Pfizer seeks to expand COVID omicron booster to 5- to 11-year-oldsIf the FDA agrees, children would start getting a kid-sized dose of the new omicron-targeted formula.
Baca lebih lajut »
 Moderna, Pfizer Seek Authorization for Children’s BoostersPfizer/BioNTech and Moderna, the two biggest COVID vaccine makers for the United States, are both seeking emergency authorization from the FDA for bivalent vaccine boosters for children.
Moderna, Pfizer Seek Authorization for Children’s BoostersPfizer/BioNTech and Moderna, the two biggest COVID vaccine makers for the United States, are both seeking emergency authorization from the FDA for bivalent vaccine boosters for children.
Baca lebih lajut »
 FDA Clears New AI Device to Aid Polyp Detection🆕 The FDA has granted 510(k) marketing clearance to a new AI–assisted polyp detection device for use during colorectal cancer screening. FDAapproves GastroTwitter
FDA Clears New AI Device to Aid Polyp Detection🆕 The FDA has granted 510(k) marketing clearance to a new AI–assisted polyp detection device for use during colorectal cancer screening. FDAapproves GastroTwitter
Baca lebih lajut »
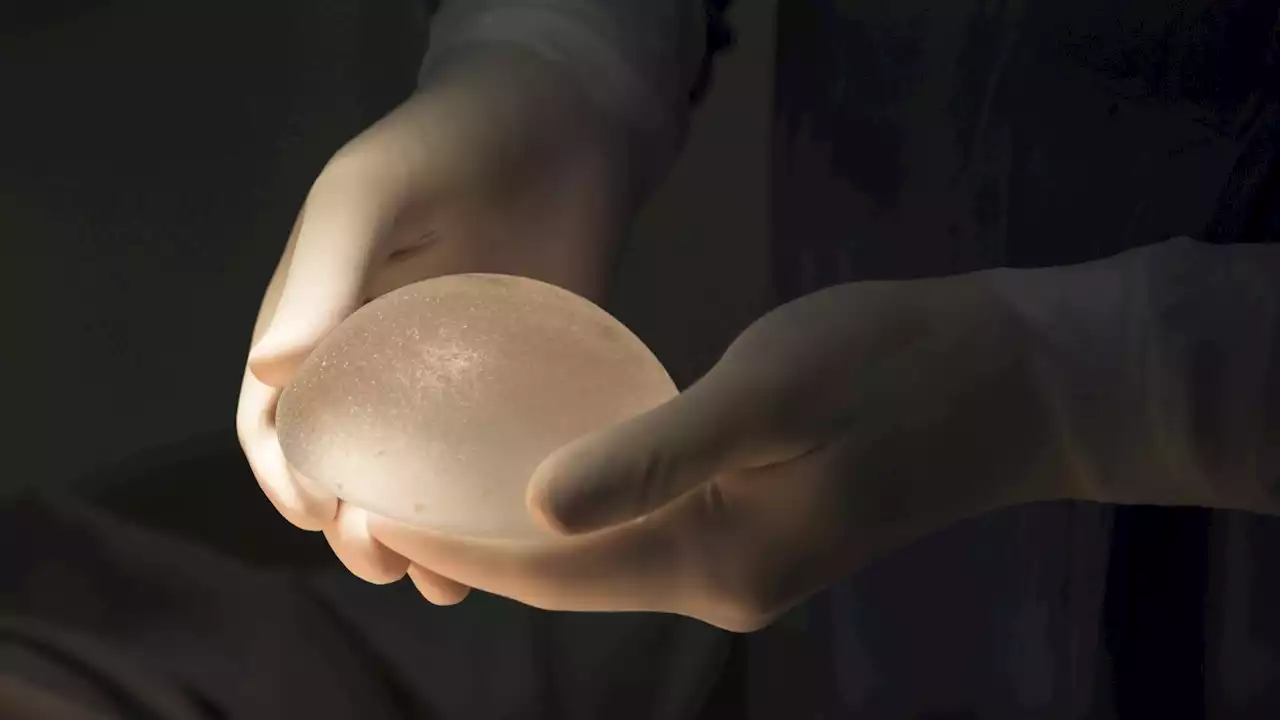 Breast implants are linked to several cancers, FDA warnsThe U.S. FDA warns that breast implants filled with silicone or saline could give rise to cancer in the scar tissue around breast implants.
Breast implants are linked to several cancers, FDA warnsThe U.S. FDA warns that breast implants filled with silicone or saline could give rise to cancer in the scar tissue around breast implants.
Baca lebih lajut »