Madrigal Pharmaceuticals Inc.’s stock rose 5% Friday after the company said it has started a rolling submission of a New Drug Application to the U.S. Food...
Madrigal Pharmaceuticals Inc.’s stock MDGL rose 5% Friday after the company said it has started a rolling submission of a New Drug Application to the U.S. Food and Drug Administration seeking accelerated approval of its resmetirom as a treatment for, nonalcoholic steatohepatitis, or
is a more severe version of nonalcoholic fatty liver disease, or NAFLD, a range of conditions that occurs when excess fat builds up in liver cells. An estimated 25% of Americans have NAFLD, while about 20% of those patients end up developing , with liver fibrosis. , in which the inflammation and cell damage caused by the fat buildup can cause cirrhosis and liver failure. The disease is one of the main reasons patients require liver transplants. Resmetirom was granted FDA Breakthrough Therapy designation in April for the treatment of
Indonesia Berita Terbaru, Indonesia Berita utama
Similar News:Anda juga dapat membaca berita serupa dengan ini yang kami kumpulkan dari sumber berita lain.
 Plano-based Reata Pharmaceuticals launches its first drug, SkyclarysPlano-based Reata Pharmaceuticals lunches its first drug Skyclarys following FDA approval. The drug is the first and only treatment for the rare...
Plano-based Reata Pharmaceuticals launches its first drug, SkyclarysPlano-based Reata Pharmaceuticals lunches its first drug Skyclarys following FDA approval. The drug is the first and only treatment for the rare...
Baca lebih lajut »
 Mira Pharmaceuticals files for IPO in busy week for dealsCannabis-focused Mira Pharmaceuticals Inc. on Thursday filed for an initial public offering, the latest in what has been a busy week for IPOs. The company,...
Mira Pharmaceuticals files for IPO in busy week for dealsCannabis-focused Mira Pharmaceuticals Inc. on Thursday filed for an initial public offering, the latest in what has been a busy week for IPOs. The company,...
Baca lebih lajut »
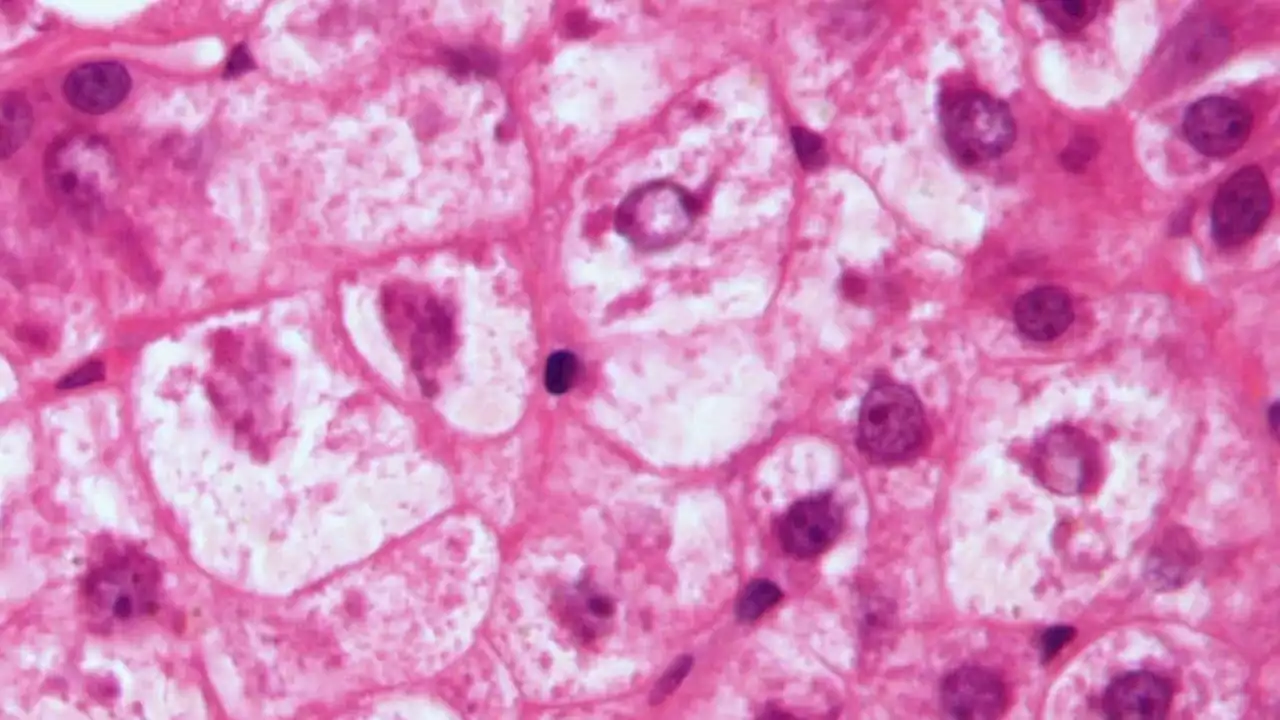 Pegozafermin Improves Fibrosis in NASH in Phase 2b StudyIn the phase 2b ENLIVEN trial, the investigational drug pegozafermin met both primary endpoints at 24 weeks and led to improvements in fibrosis, paving the way for phase 3 development.
Pegozafermin Improves Fibrosis in NASH in Phase 2b StudyIn the phase 2b ENLIVEN trial, the investigational drug pegozafermin met both primary endpoints at 24 weeks and led to improvements in fibrosis, paving the way for phase 3 development.
Baca lebih lajut »
 Merus stock jumps 10% after FDA’s action on cancer drugShares of Merus NV rallied as much as 10% in the extended session Thursday after the pharma company said the U.S. Food and Drug Administration has granted...
Merus stock jumps 10% after FDA’s action on cancer drugShares of Merus NV rallied as much as 10% in the extended session Thursday after the pharma company said the U.S. Food and Drug Administration has granted...
Baca lebih lajut »
 Gonorrhea shot gets FDA fast track as resistant cases multiplyThe fast-track designation puts the shot in position to become the first preventive for a common, often-undetected infection that’s gaining resistance to treatment.
Gonorrhea shot gets FDA fast track as resistant cases multiplyThe fast-track designation puts the shot in position to become the first preventive for a common, often-undetected infection that’s gaining resistance to treatment.
Baca lebih lajut »
 FDA to ‘Fast Track’ Review of Experimental Gonorrhea VaccineThe FDA announced on Tuesday that it will 'fast track' review of a vaccine in development to prevent gonorrhea infections.
FDA to ‘Fast Track’ Review of Experimental Gonorrhea VaccineThe FDA announced on Tuesday that it will 'fast track' review of a vaccine in development to prevent gonorrhea infections.
Baca lebih lajut »
