Leqembi is the first Alzheimer's antibody treatment to receive full FDA approval.
The closely watched drug has prompted debate between medical and market experts.that the treatment of the drug, specifically the slowing in the progression of the illness, falls below the level that would be considered "noticeable" to a patient.
"The odds for brain swelling and hemorrhage are far higher than any actual improvement," Espay, who launched a petition in June calling for the Alzheimer's treatment to not get full approval, told NBC News."This treatment is safe and effective for Alzheimer's disease," Cheung said Thursday. "The benefit risk profile is well established from the large late stage clinical trial and we believe in year three, about 100,000 individuals could be diagnosed and eligible for this important treatment," he said.
Indonesia Berita Terbaru, Indonesia Berita utama
Similar News:Anda juga dapat membaca berita serupa dengan ini yang kami kumpulkan dari sumber berita lain.
 FDA approves use of Leqembi to treat Alzheimer's patientsFDA approves use of Leqembi Alzheimer\u0027s patients
FDA approves use of Leqembi to treat Alzheimer's patientsFDA approves use of Leqembi Alzheimer\u0027s patients
Baca lebih lajut »
 FDA to grant full approval for Alzheimer's medication LecanemabIf the FDA grants Lecanemab full approval, medical experts say Medicaid and Medicare will likely cover it MORE ⬇️
FDA to grant full approval for Alzheimer's medication LecanemabIf the FDA grants Lecanemab full approval, medical experts say Medicaid and Medicare will likely cover it MORE ⬇️
Baca lebih lajut »
 FDA to decide on Alzheimer’s drug approvalFull approval would likely prompt the Centers for Medicare and Medicaid Services to change how it covers the drug, broadening access for up to about a million people with early onset Alzheimer’s.
FDA to decide on Alzheimer’s drug approvalFull approval would likely prompt the Centers for Medicare and Medicaid Services to change how it covers the drug, broadening access for up to about a million people with early onset Alzheimer’s.
Baca lebih lajut »
 FDA to decide on Alzheimer’s drug approvalFull approval would likely prompt the Centers for Medicare and Medicaid Services to change how it covers the drug, broadening access for up to about a million people with early onset Alzheimer’s.
FDA to decide on Alzheimer’s drug approvalFull approval would likely prompt the Centers for Medicare and Medicaid Services to change how it covers the drug, broadening access for up to about a million people with early onset Alzheimer’s.
Baca lebih lajut »
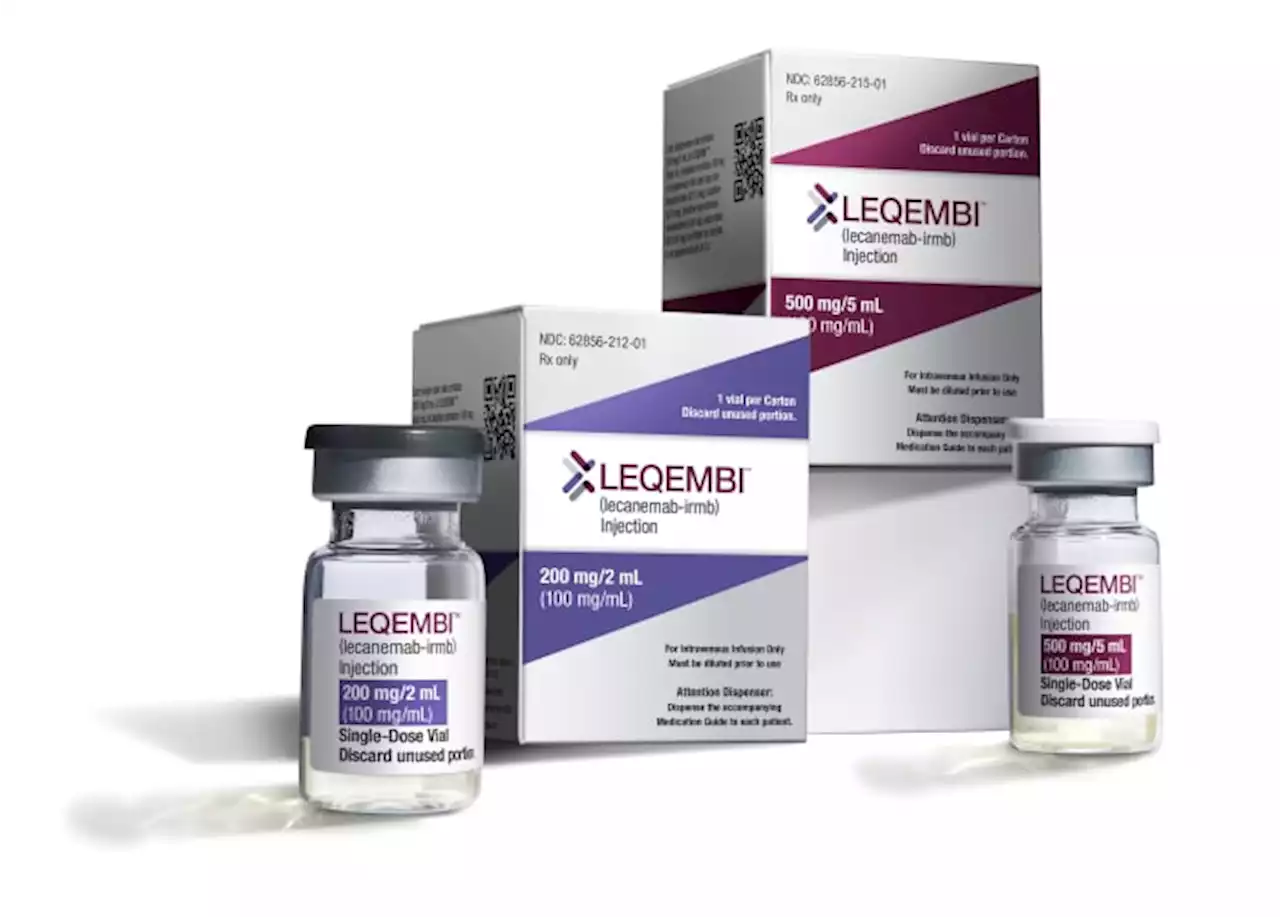 FDA to make a decision on whether to approve first Alzheimer’s drugThe FDA is set to decide whether it will fully approve the first Alzheimer’s drug to show it could slow the disease’s progression in certain patients. But the decision could also have other implications, including who would get access to it.
FDA to make a decision on whether to approve first Alzheimer’s drugThe FDA is set to decide whether it will fully approve the first Alzheimer’s drug to show it could slow the disease’s progression in certain patients. But the decision could also have other implications, including who would get access to it.
Baca lebih lajut »
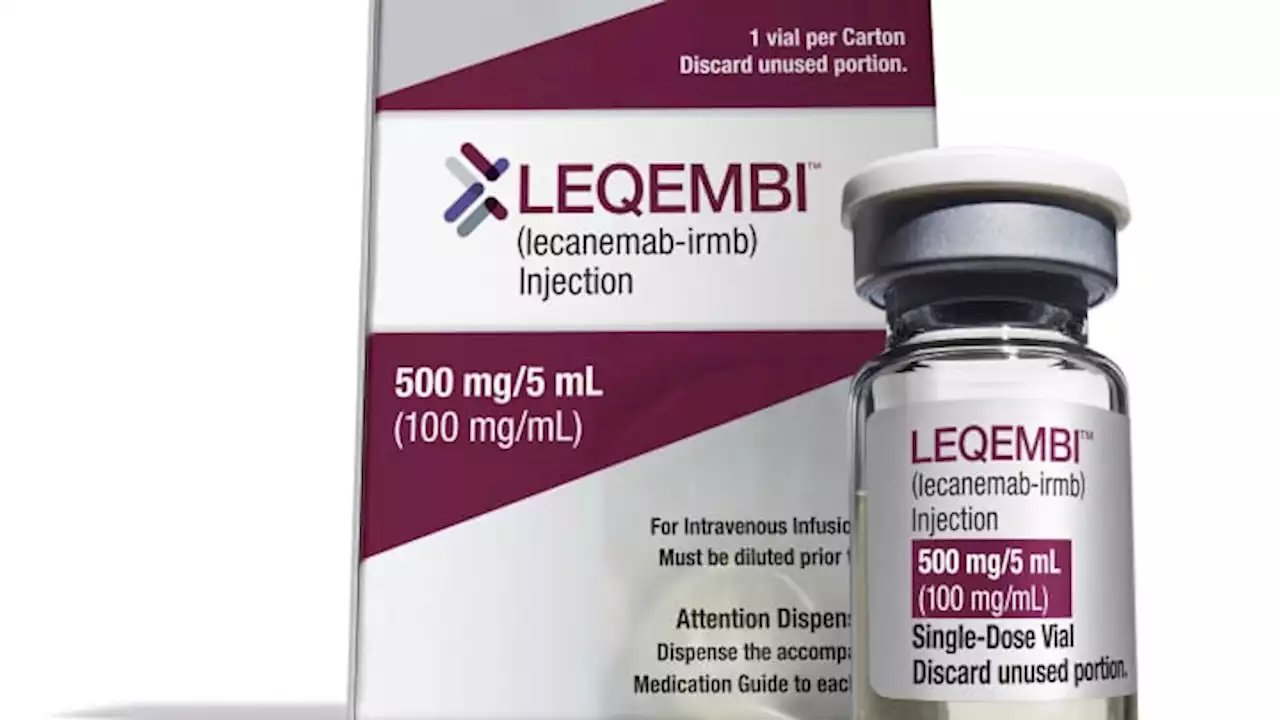 FDA approves Alzheimer’s drug Leqembi, paving way for broader Medicare coverageLeqembi slowed cognitive decline in a clinical trial, but the treatment is expensive and carries serious risks of brain swelling and bleeding.
FDA approves Alzheimer’s drug Leqembi, paving way for broader Medicare coverageLeqembi slowed cognitive decline in a clinical trial, but the treatment is expensive and carries serious risks of brain swelling and bleeding.
Baca lebih lajut »
