The U.S. Food and Drug Administration said on Thursday it will require new safety warnings to be added in the prescribing information on labels for opioid pain relievers, including a warning about increased sensitivity to pain.
FDA said data suggests patients who use opioids for pain relief after surgery often have leftover tablets, which puts them at risk for addiction and overdose.safety warnings for these drugs will provide clarity about which patients opioid pain drugs should be prescribed to and the appropriate dosage and administration, the health regulator said.
Among other changes, the new labeling would also have to carry a warning that risk of overdose increases with higher dosage and that immediate-release opioids should not be used for an extended period unless a patient's pain remains severe.Nucynta are among opioid pain relievers currently sold in the United States.
Indonesia Berita Terbaru, Indonesia Berita utama
Similar News:Anda juga dapat membaca berita serupa dengan ini yang kami kumpulkan dari sumber berita lain.
 Court Partially Halts Ruling Restricting Access to Abortion PillA hardline conservative judge suspended the FDA’s approval of the drug last Friday.
Court Partially Halts Ruling Restricting Access to Abortion PillA hardline conservative judge suspended the FDA’s approval of the drug last Friday.
Baca lebih lajut »
 US FDA declines to approve Eli Lilly's bowel disease drugEli Lilly and Co said on Thursday that the U.S. Food and Drug Administration (FDA) had declined to approve its drug to treat a type of chronic inflammatory bowel disease in adults.
US FDA declines to approve Eli Lilly's bowel disease drugEli Lilly and Co said on Thursday that the U.S. Food and Drug Administration (FDA) had declined to approve its drug to treat a type of chronic inflammatory bowel disease in adults.
Baca lebih lajut »
 FDA Declines to Approve Eli Lilly's Bowel Disease DrugEli Lilly said on Thursday that the FDA had declined to approve its drug to treat a type of chronic inflammatory bowel disease in adults.
FDA Declines to Approve Eli Lilly's Bowel Disease DrugEli Lilly said on Thursday that the FDA had declined to approve its drug to treat a type of chronic inflammatory bowel disease in adults.
Baca lebih lajut »
 Court preserves access to abortion drug, tightens rulesA federal appeals court has preserved access to an abortion drug for now but under tighter rules that would allow the drug only to be dispensed up to seven weeks, not 10, and not by mail. The drug, mifepristone, was approved for use by the Food and Drug Administration more than two decades ago. It’s used in combination with a second drug, misoprostol. The 5th U.S. Circuit Court of Appeals in New Orleans ruled Wednesday just before midnight. By a 2-1 vote a panel of three judges narrowed for now a decision by a lower court judge in Texas that had completely blocked the FDA’s approval of the drug following a lawsuit by mifepristone’s opponents.
Court preserves access to abortion drug, tightens rulesA federal appeals court has preserved access to an abortion drug for now but under tighter rules that would allow the drug only to be dispensed up to seven weeks, not 10, and not by mail. The drug, mifepristone, was approved for use by the Food and Drug Administration more than two decades ago. It’s used in combination with a second drug, misoprostol. The 5th U.S. Circuit Court of Appeals in New Orleans ruled Wednesday just before midnight. By a 2-1 vote a panel of three judges narrowed for now a decision by a lower court judge in Texas that had completely blocked the FDA’s approval of the drug following a lawsuit by mifepristone’s opponents.
Baca lebih lajut »
 Latin and Tex-Mex overtake Italian as America's go-to food orderFor the longest time, Italian food was America's favorite, but increasingly it's Latin American and Tex-Mex food.
Latin and Tex-Mex overtake Italian as America's go-to food orderFor the longest time, Italian food was America's favorite, but increasingly it's Latin American and Tex-Mex food.
Baca lebih lajut »
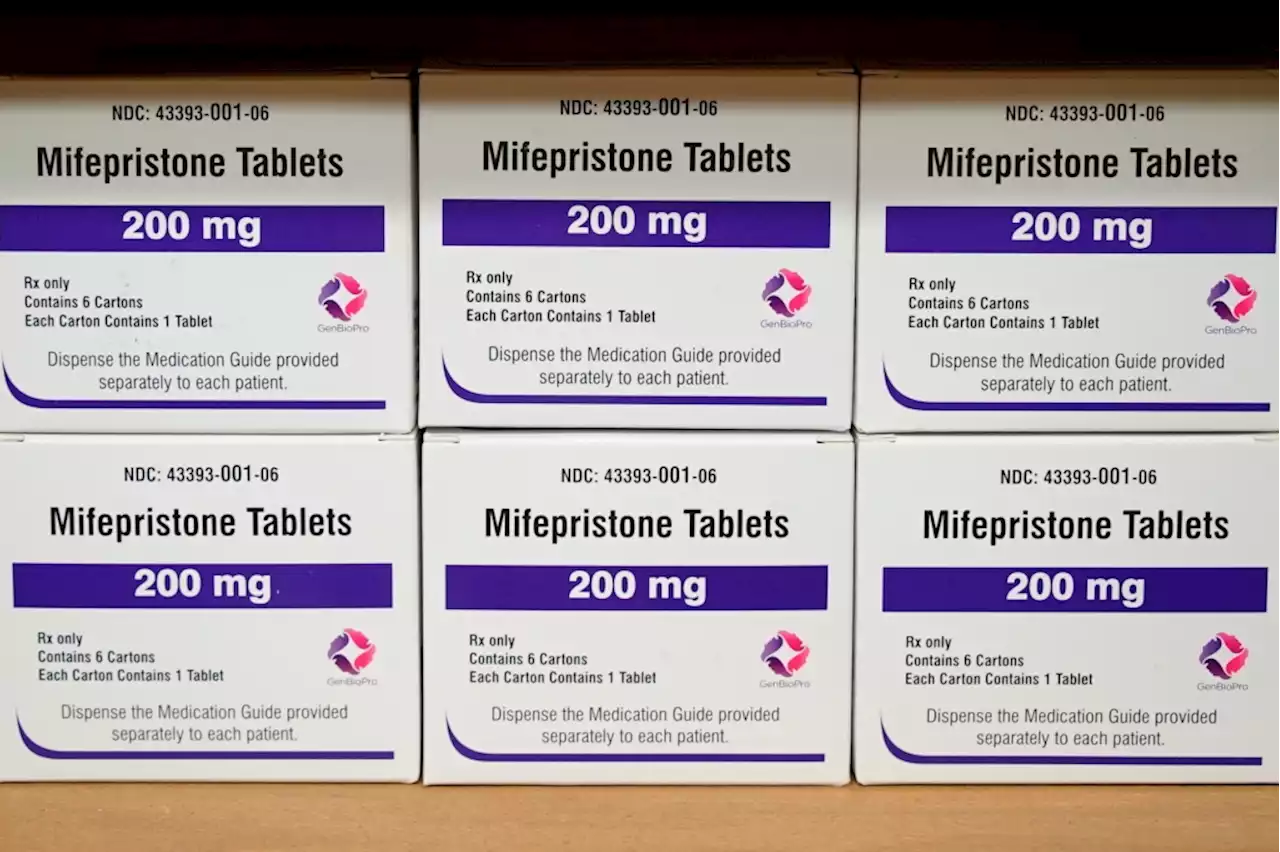 Appeals court partially freezes Texas judge’s order that would have suspended FDA approval of medication abortion pillA federal appeals court late Wednesday night froze parts of a Texas judge’s order that would have suspended the US Food and Drug Administration’s approval of a medication abortion drug.
Appeals court partially freezes Texas judge’s order that would have suspended FDA approval of medication abortion pillA federal appeals court late Wednesday night froze parts of a Texas judge’s order that would have suspended the US Food and Drug Administration’s approval of a medication abortion drug.
Baca lebih lajut »
