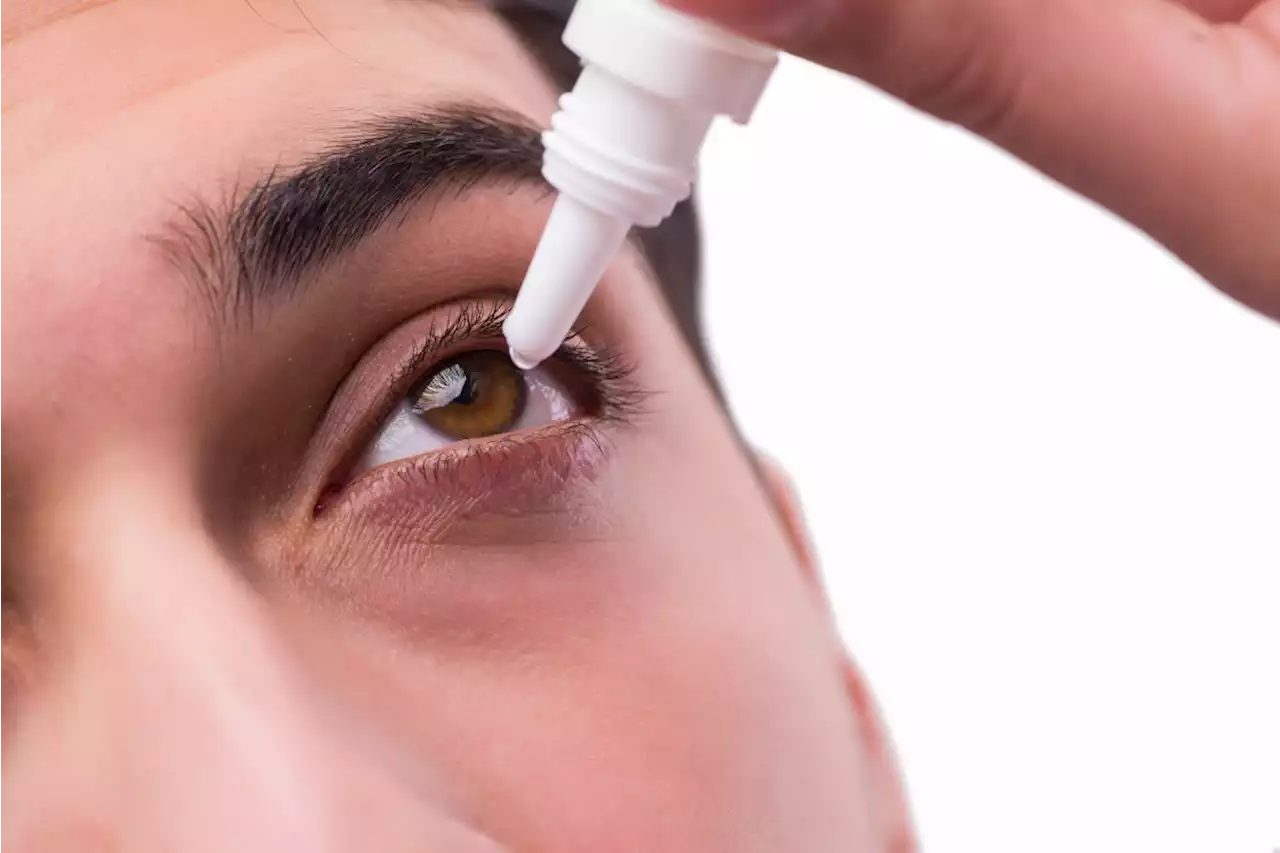
The FDA is “warning consumers and health care professionals not to purchase or use Delsam Pharma’s Artificial Eye Ointment due to potential bacterial contamination,” the agency announced Tuesday.…
Muri Assunção | New York Daily News
The ointment, which is manufactured by Global Pharma Healthcare Private Limited, is a lubricant used to prevent irritation and relieve dryness of the eye, according to a description on Delsam Pharma’s website. “Patients who have signs or symptoms of an eye infection should talk to their health care provider or seek medical care immediately,” the agency added.
Indonesia Berita Terbaru, Indonesia Berita utama
Similar News:Anda juga dapat membaca berita serupa dengan ini yang kami kumpulkan dari sumber berita lain.
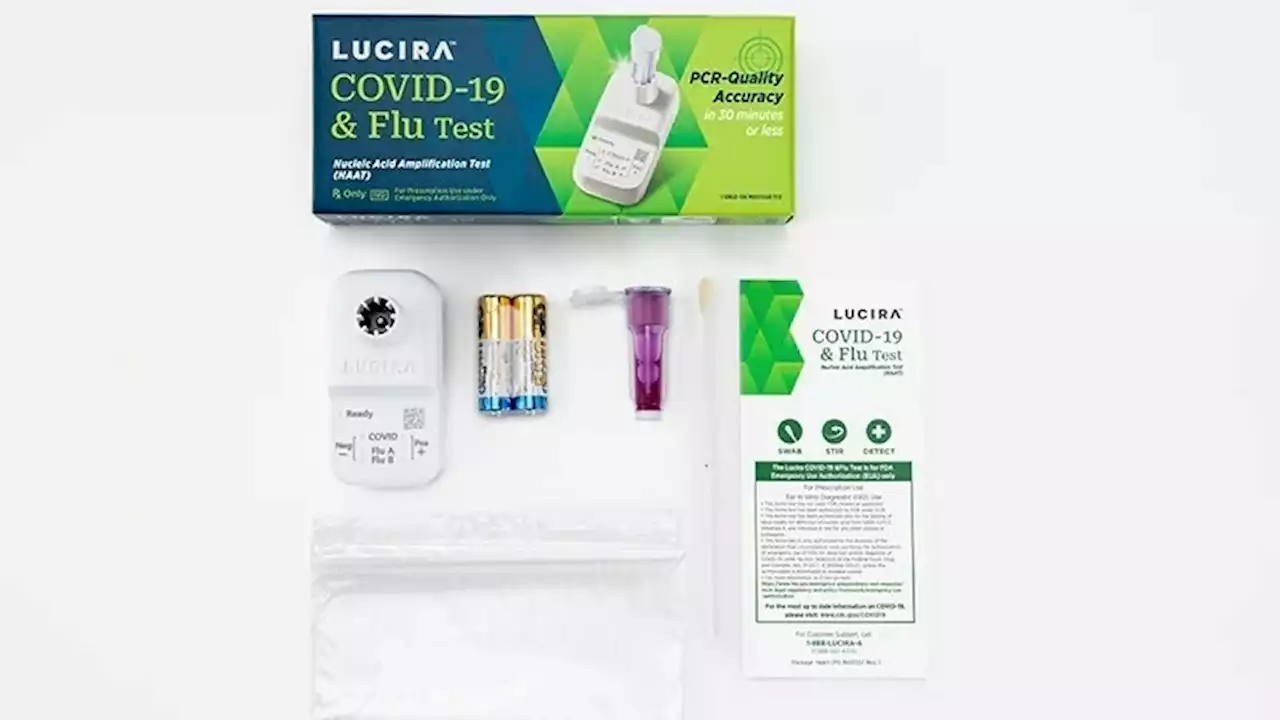 FDA authorizes the first at-home test for COVID-19 and the fluThe FDA has authorized the first at-home test that can simultaneously detect both COVID and the flu. The nasal swab provides results within 30 minutes.
FDA authorizes the first at-home test for COVID-19 and the fluThe FDA has authorized the first at-home test that can simultaneously detect both COVID and the flu. The nasal swab provides results within 30 minutes.
Baca lebih lajut »
 PolitiFact - FDA approval is required for COVID-19 vaccine manufacturers to change ingredients in shotsThe COVID-19 vaccines from Pfizer and Moderna are no longer under an emergency use authorization for adults. Even if they were, any changes would require the manufacturers to submit data and have their product reassessed by the FDA.
PolitiFact - FDA approval is required for COVID-19 vaccine manufacturers to change ingredients in shotsThe COVID-19 vaccines from Pfizer and Moderna are no longer under an emergency use authorization for adults. Even if they were, any changes would require the manufacturers to submit data and have their product reassessed by the FDA.
Baca lebih lajut »
 First at-home combination test for COVID and flu authorized by FDAThe FDA has authorized the first over-the-counter at-home test that can detect and differentiate between a test result for flu and one for COVID-19.
First at-home combination test for COVID and flu authorized by FDAThe FDA has authorized the first over-the-counter at-home test that can detect and differentiate between a test result for flu and one for COVID-19.
Baca lebih lajut »
 Democrat State AGs Sue FDA Over Abortion Pill RestrictionsSeveral Democrat state attorneys general filed a lawsuit on Friday against the U.S. Food and Drug Administration (FDA), challenging the agency’s restrictions on mifepristone, the the first of two drugs used in a medication abortion.
Democrat State AGs Sue FDA Over Abortion Pill RestrictionsSeveral Democrat state attorneys general filed a lawsuit on Friday against the U.S. Food and Drug Administration (FDA), challenging the agency’s restrictions on mifepristone, the the first of two drugs used in a medication abortion.
Baca lebih lajut »