U.S. regulators have cleared doses of the updated COVID-19 vaccines to children younger than 5
Deborah Sampson, left, a nurse at a University of Washington Medical Center clinic in Seattle, gives a Pfizer COVID-19 vaccine shot to a 20-month-old child, June 21, 2022, in Seattle. The U.S. on Thursday, Dec. 8, 2022 open doses of the updated COVID-19 vaccines for most children younger than age 5.
The FDA now has cleared their use in tots starting at age 6 months -- but just who is eligible depends on what vaccinations they've already had, and which kind. Few youngsters have gotten the full primary series since shots for the littlest kids--Children under age 6 who’ve already gotten two original doses of Moderna’s COVID-19 vaccine can get a single booster of Moderna’s updated formula if it's been at least two months since their last shot.
--Children under 5 who already got all three Pfizer doses aren’t yet eligible for an updated booster. Data expected next month should help the FDA determine if and when those tots need the omicron-targeted booster. Just 3% of tots under 2 and nearly 5% of those 2 to 4 have gotten their primary doses so far, according to the CDC.
Indonesia Berita Terbaru, Indonesia Berita utama
Similar News:Anda juga dapat membaca berita serupa dengan ini yang kami kumpulkan dari sumber berita lain.
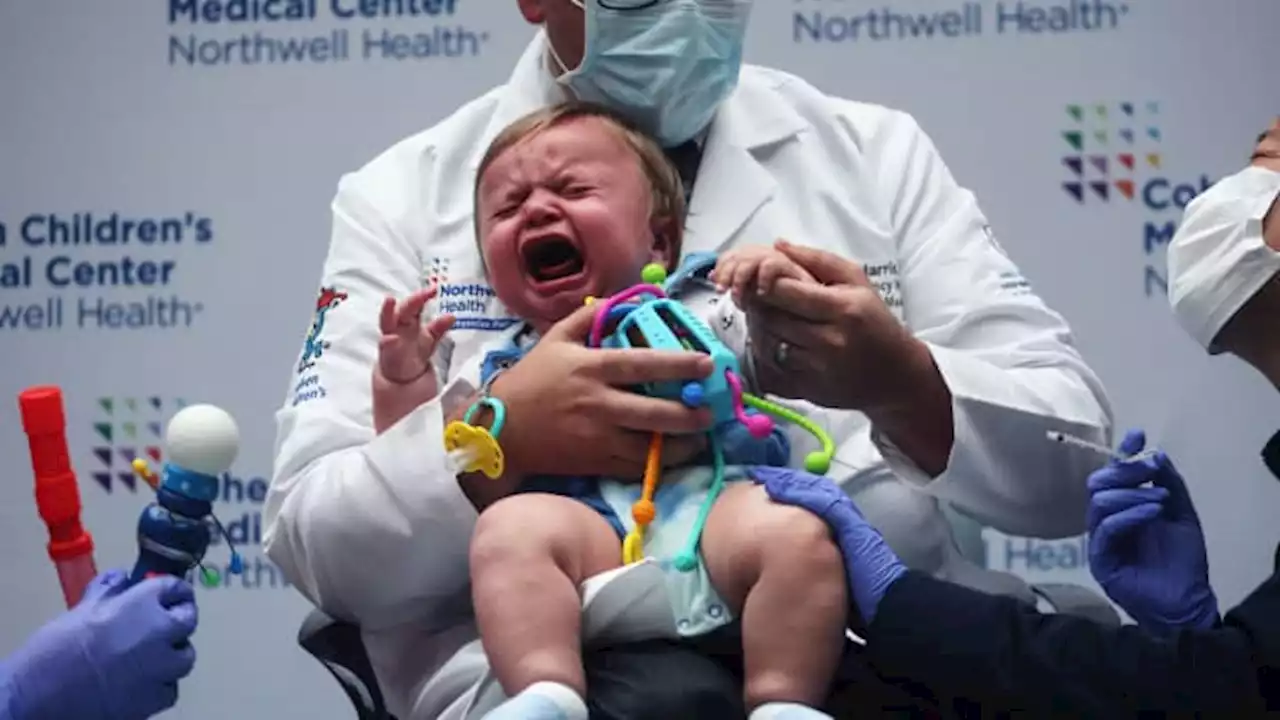 FDA authorizes Covid omicron vaccines for children as young as 6 months old
FDA authorizes Covid omicron vaccines for children as young as 6 months old
Baca lebih lajut »
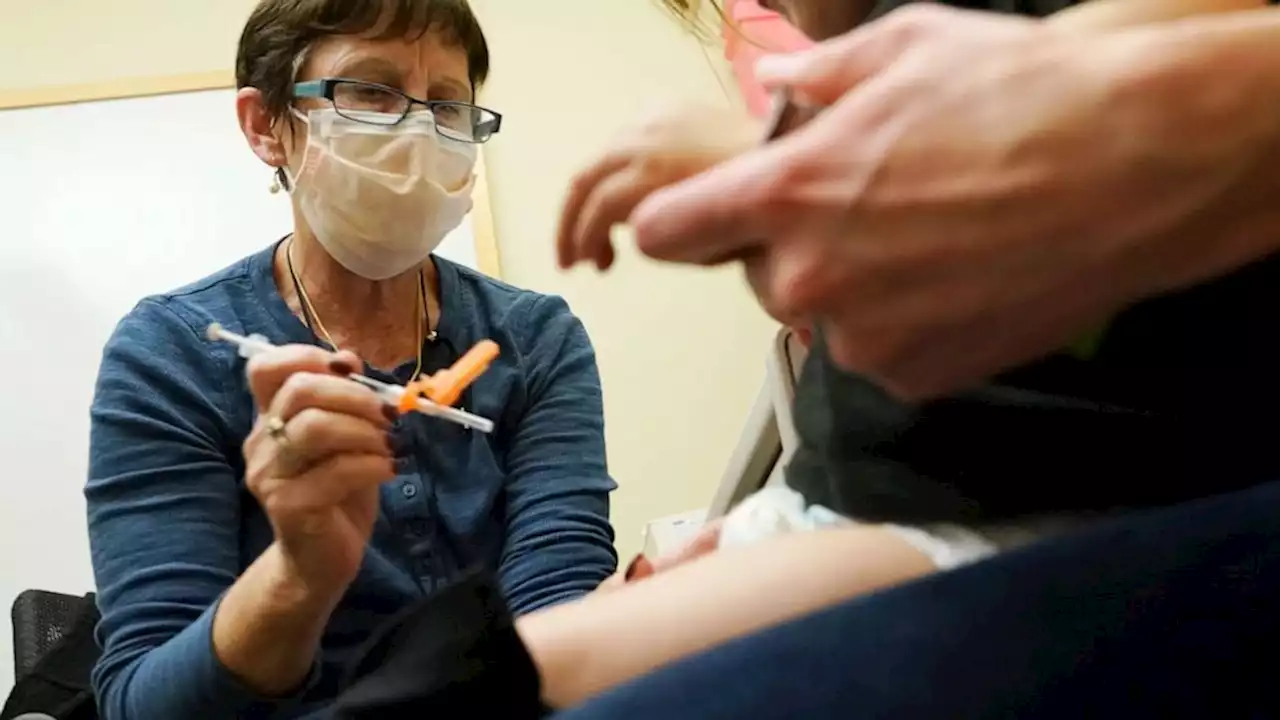 FDA authorizes updated COVID-19 boosters for kids under 5The U.S. Food and Drug Administration authorized bivalent COVID-19 boosters for children between ages 6 months and 4 years Thursday.
FDA authorizes updated COVID-19 boosters for kids under 5The U.S. Food and Drug Administration authorized bivalent COVID-19 boosters for children between ages 6 months and 4 years Thursday.
Baca lebih lajut »
 Pfizer partners with Clear Creek Bio to develop oral COVID-19 drugPfizer Inc and Clear Creek Bio Inc on Tuesday announced a collaboration to identify a potential drug candidate and develop a new class of oral treatment against COVID-19, as Pfizer seeks to expand its anti-infective pipeline.
Pfizer partners with Clear Creek Bio to develop oral COVID-19 drugPfizer Inc and Clear Creek Bio Inc on Tuesday announced a collaboration to identify a potential drug candidate and develop a new class of oral treatment against COVID-19, as Pfizer seeks to expand its anti-infective pipeline.
Baca lebih lajut »
 The flu, COVID-19 and RSV: Why health experts are concerned about this ‘tripledemic’Hospitals are filling up with people battling both flu and COVID as well as with pediatric patients suffering from RSV. This is what health experts have deemed as the ‘tripledemic’ and there’s concern that these infections will continue to spread.
The flu, COVID-19 and RSV: Why health experts are concerned about this ‘tripledemic’Hospitals are filling up with people battling both flu and COVID as well as with pediatric patients suffering from RSV. This is what health experts have deemed as the ‘tripledemic’ and there’s concern that these infections will continue to spread.
Baca lebih lajut »
 COVID-19 surge continues building in LA County, with 3,100 new casesAccording to state figures, there were 1,270 COVID-positive patients in county hospitals as of Tuesday, up from 1,205 reported on Saturday.
COVID-19 surge continues building in LA County, with 3,100 new casesAccording to state figures, there were 1,270 COVID-positive patients in county hospitals as of Tuesday, up from 1,205 reported on Saturday.
Baca lebih lajut »
