The head of the Food and Drug Administration is preparing to tell lawmakers about events that led to a months-long gap before inspecting the plant at the center of a nationwide baby formula shortage.
Califf will tell lawmakers that the FDA began planning to visit the Sturgis, Michigan, plant in early December, with inspectors set to arrive on Dec. 30. But Abbott said that about a dozen of its employees had recently tested positive for COVID-19 and requested a delay. As a result, the FDA didn't begin its inspection until Jan. 31.
“Because it was a negotiation process with a regulated firm, the U.S. government did not completely control the timeline,” states Califf's written testimony. Several FDA staffers reviewed the complaint in late October, but officials didn't request an interview until early December. Because of conflicts with the whistleblower's schedule, the interview didn't take place until Dec. 22, according to the FDA testimony.
Indonesia Berita Terbaru, Indonesia Berita utama
Similar News:Anda juga dapat membaca berita serupa dengan ini yang kami kumpulkan dari sumber berita lain.
 U.N. rights chief visits China as police files detail abuse of UyghursMichelle Bachelet's trip comes as 'Xinjiang Police Files' on the internment of Uyghurs and other minorities in China become public.
U.N. rights chief visits China as police files detail abuse of UyghursMichelle Bachelet's trip comes as 'Xinjiang Police Files' on the internment of Uyghurs and other minorities in China become public.
Baca lebih lajut »
 Opinion | Why Is the FDA Seizing Baby Formula During a Baby Formula Shortage?From WSJopinion: The FDA’s actions have exacerbated the baby formula shortage problem they were trying to solve, write scottlincicome and emilyekins
Opinion | Why Is the FDA Seizing Baby Formula During a Baby Formula Shortage?From WSJopinion: The FDA’s actions have exacerbated the baby formula shortage problem they were trying to solve, write scottlincicome and emilyekins
Baca lebih lajut »
 FDA head grilled by members of both parties on baby formula shortage crisisFood and Drug Administration Commissioner Robert Califf took criticism from both Democrats and Republicans on a House subcommittee over the baby formula shortage on his watch that has left many parents desperate.
FDA head grilled by members of both parties on baby formula shortage crisisFood and Drug Administration Commissioner Robert Califf took criticism from both Democrats and Republicans on a House subcommittee over the baby formula shortage on his watch that has left many parents desperate.
Baca lebih lajut »
 Pfizer Says 3 COVID Shots Protect Kids Under 5, Will Soon Send Data to FDAThe 18 million kids under 5 are the only group in the U.S. not yet eligible for COVID-19 vaccination.
Pfizer Says 3 COVID Shots Protect Kids Under 5, Will Soon Send Data to FDAThe 18 million kids under 5 are the only group in the U.S. not yet eligible for COVID-19 vaccination.
Baca lebih lajut »
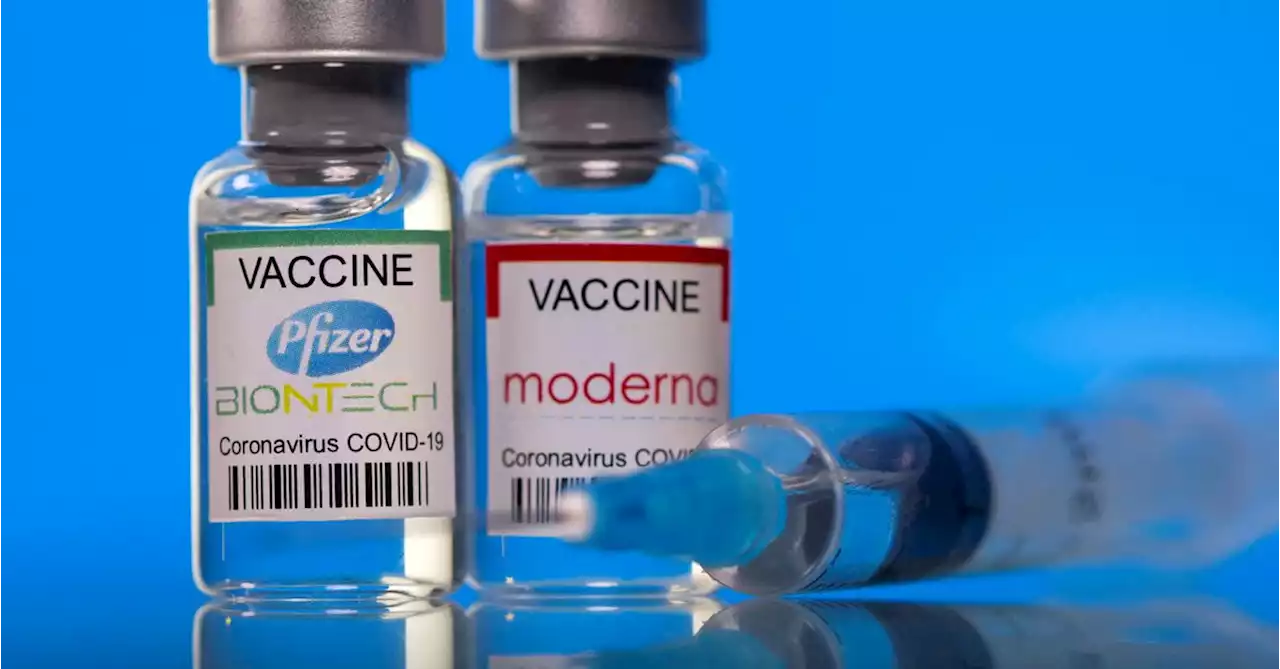 U.S. FDA sets June meeting dates for Moderna, Pfizer small children COVID-19 vaccinesThe U.S. Food and Drug Administration set June 14-15 as the new meeting date to review Moderna Inc's emergency authorization request for its COVID-19 vaccine for children aged 6 months to 5 years and Pfizer Inc's vaccine for those aged 6 months through 4 years.
U.S. FDA sets June meeting dates for Moderna, Pfizer small children COVID-19 vaccinesThe U.S. Food and Drug Administration set June 14-15 as the new meeting date to review Moderna Inc's emergency authorization request for its COVID-19 vaccine for children aged 6 months to 5 years and Pfizer Inc's vaccine for those aged 6 months through 4 years.
Baca lebih lajut »
 Jif peanut butter recall: Throw away these jars right now, FDA warnsThe J.M. Smucker Company, Jif peanut butter's parent company, has issued a nationwide recall of its product due to potential Salmonella contamination.
Jif peanut butter recall: Throw away these jars right now, FDA warnsThe J.M. Smucker Company, Jif peanut butter's parent company, has issued a nationwide recall of its product due to potential Salmonella contamination.
Baca lebih lajut »
