Children ages 5 to 11 will soon be eligible to receive an updated COVID-19 booster targeting the omicron variant after the Food and Drug Administration authorized expanding eligibility to younger school-age children on Wednesday.
The agency authorized Pfizer's updated booster for children ages 5-11 and Moderna's shot for children ages 6-17 for at least two months following the completion of their primary 2-dose vaccination series or last booster."While it has largely been the case that COVID-19 tends to be less severe in children than adults, as the various waves of COVID-19 have occurred, more children have gotten sick with the disease and have been hospitalized," said Dr.
The Centers for Disease Control and Prevention needs to sign off on the authorization before providers can administer the updated booster to younger age groups, though the agency signaled last month it intends to recommend the shots. In August, the FDA authorized the new boosters from Moderna for people 18 and older and Pfizer-BioNTech's booster candidate for people 12 years and older. As of Oct. 6, 11.5 million people have received an updated booster, representing only a fraction of the population that is eligible for the shots, according to the CDC.
Booster vaccinations for younger age groups could have a slow uptick as well, as only 31% of children ages 5-11 have received their primary vaccination series, according to the American Academy of Pediatrics.The Biden administration has encouraged the updated boosters, which target both the original strain of the virus from 2020 and omicron subvariants BA.4 and BA.5, anticipating a rise in cases as people spend more time indoors in the fall and winter and going into the holiday season.
Indonesia Berita Terbaru, Indonesia Berita utama
Similar News:Anda juga dapat membaca berita serupa dengan ini yang kami kumpulkan dari sumber berita lain.
 FDA authorizes updated COVID-19 boosters for children as young as 5The updated shots contain half the recipe that targeted the original coronavirus strain and half protection against the dominant BA.4 and BA.5 omicron versions.
FDA authorizes updated COVID-19 boosters for children as young as 5The updated shots contain half the recipe that targeted the original coronavirus strain and half protection against the dominant BA.4 and BA.5 omicron versions.
Baca lebih lajut »
 FDA authorizes updated COVID-19 boosters for children as young as 5The updated shots contain half the recipe that targeted the original coronavirus strain and half protection against the dominant BA.4 and BA.5 omicron versions.
FDA authorizes updated COVID-19 boosters for children as young as 5The updated shots contain half the recipe that targeted the original coronavirus strain and half protection against the dominant BA.4 and BA.5 omicron versions.
Baca lebih lajut »
 FDA authorizes updated bivalent COVID-19 booster for children aged 5 and olderThe Food and Drug Administration authorized the updated bivalent COVID-19 booster for children ages 5 and older Monday.
FDA authorizes updated bivalent COVID-19 booster for children aged 5 and olderThe Food and Drug Administration authorized the updated bivalent COVID-19 booster for children ages 5 and older Monday.
Baca lebih lajut »
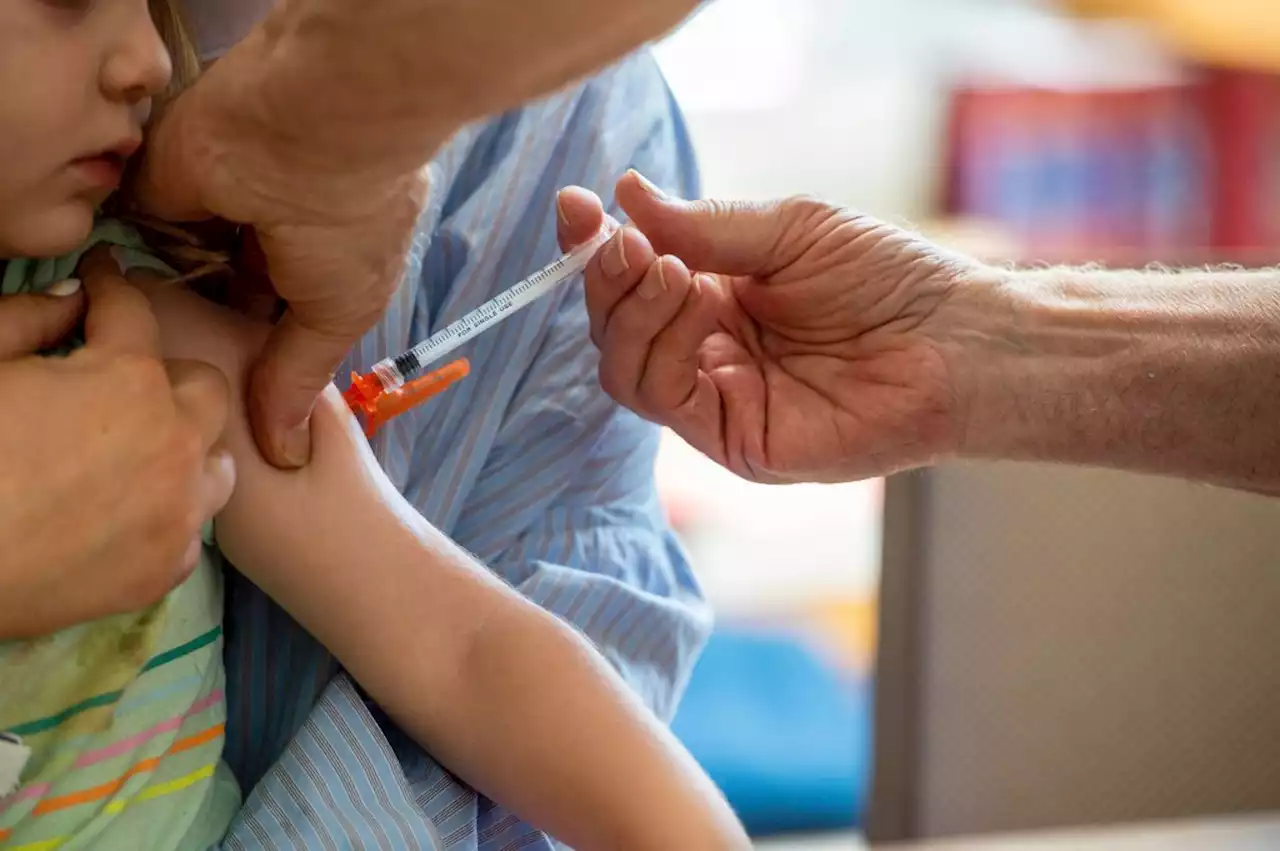 FDA authorizes updated Pfizer and Moderna Covid-19 booster shots for children as young as 5Like the boosters that became available for people 12 and older in September, these bivalent boosters target the original coronavirus strain as well as the Omicron BA.4/BA.5 subvariants.
FDA authorizes updated Pfizer and Moderna Covid-19 booster shots for children as young as 5Like the boosters that became available for people 12 and older in September, these bivalent boosters target the original coronavirus strain as well as the Omicron BA.4/BA.5 subvariants.
Baca lebih lajut »
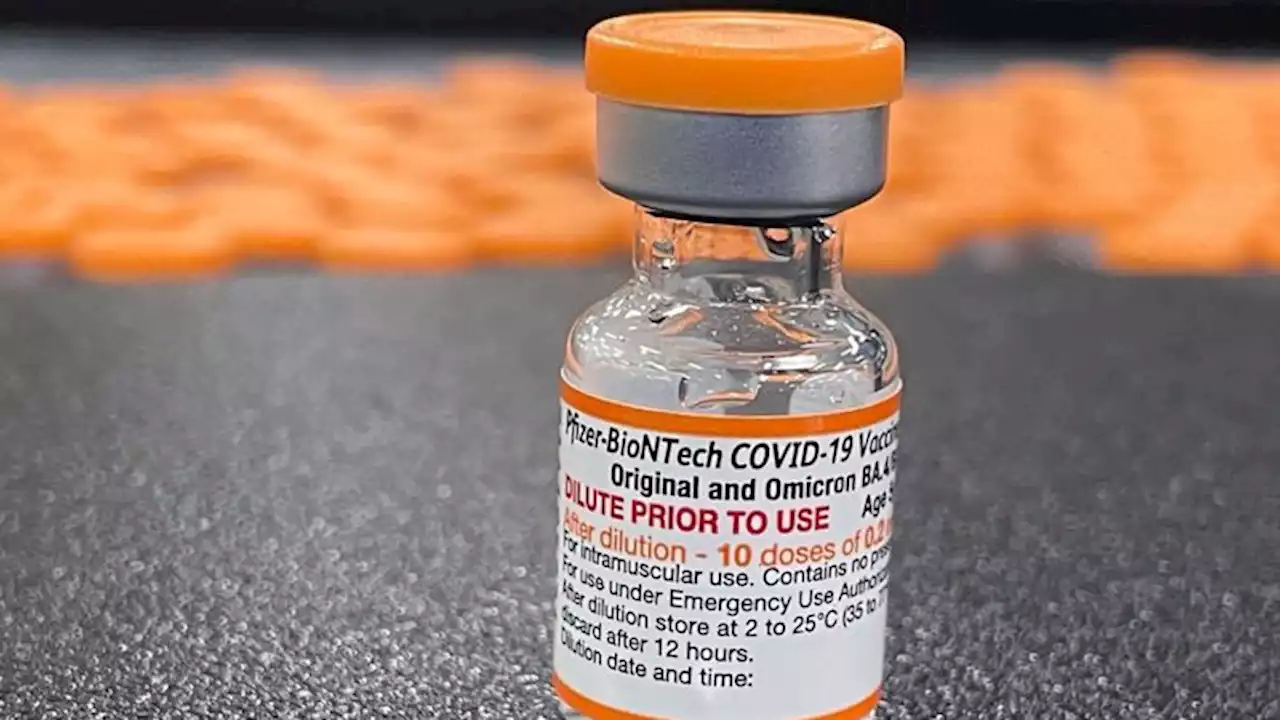 FDA authorizes updated Covid-19 booster shots for children as young as 5 | CNNFDA authorizes updated Covid-19 booster shots for children as young as 5. The boosters target the original coronavirus strain and Omicron subvariants.
FDA authorizes updated Covid-19 booster shots for children as young as 5 | CNNFDA authorizes updated Covid-19 booster shots for children as young as 5. The boosters target the original coronavirus strain and Omicron subvariants.
Baca lebih lajut »
