The Food and Drug Administration has granted Novavax's COVID-19 vaccine emergency use authorization.
The Food and Drug Administration has granted Novavax's COVID-19 vaccine emergency use authorization, paving the way for a new fourth option for the"Today's authorization offers adults in the United States who have not yet received a COVID-19 vaccine another option that meets the FDA's rigorous standards for safety, effectiveness and manufacturing quality needed to support emergency use authorization," said FDA Commissioner Dr.
"The American public can trust that this vaccine, like all vaccines that are used in the United States, has undergone the FDA's rigorous and comprehensive scientific and regulatory review," said the FDA's Dr. Peter Marks.
"What really took the longest time, however, wasn't the manufacturing of the product. It was the generation of the assays to illustrate that we could make the product over and over again, the same way, and to deploy those assays against the multiple lots," said Novavax's Filip Dubovsky. Some 3.2 million doses of Novavax's vaccine have been secured by the Biden administration. The company is among the original roster of Operation Warp Speed contracted vaccinesBut it's unclear when or how many of those doses will be initially available for states and pharmacies to order.
Indonesia Berita Terbaru, Indonesia Berita utama
Similar News:Anda juga dapat membaca berita serupa dengan ini yang kami kumpulkan dari sumber berita lain.
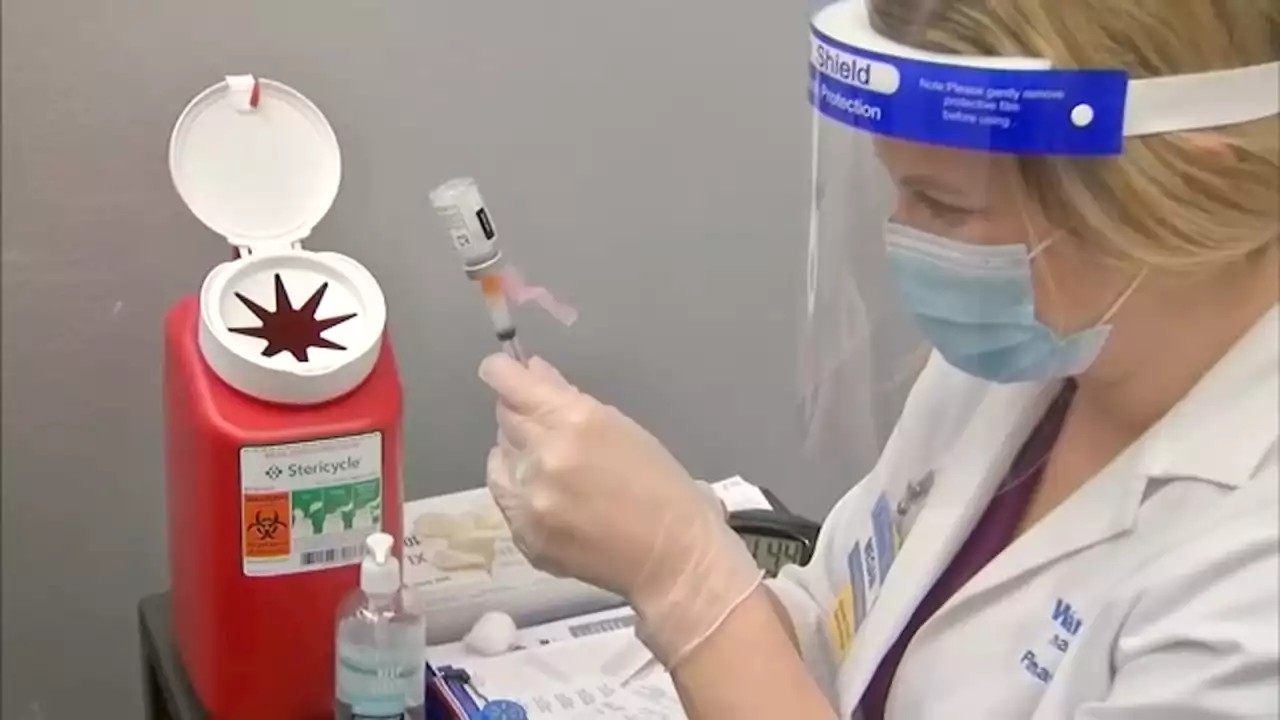 'COVID is not done with us': Why is the BA.5 COVID-19 variant so contagious?The COVID-19 pandemic is far from over, according to experts.
'COVID is not done with us': Why is the BA.5 COVID-19 variant so contagious?The COVID-19 pandemic is far from over, according to experts.
Baca lebih lajut »
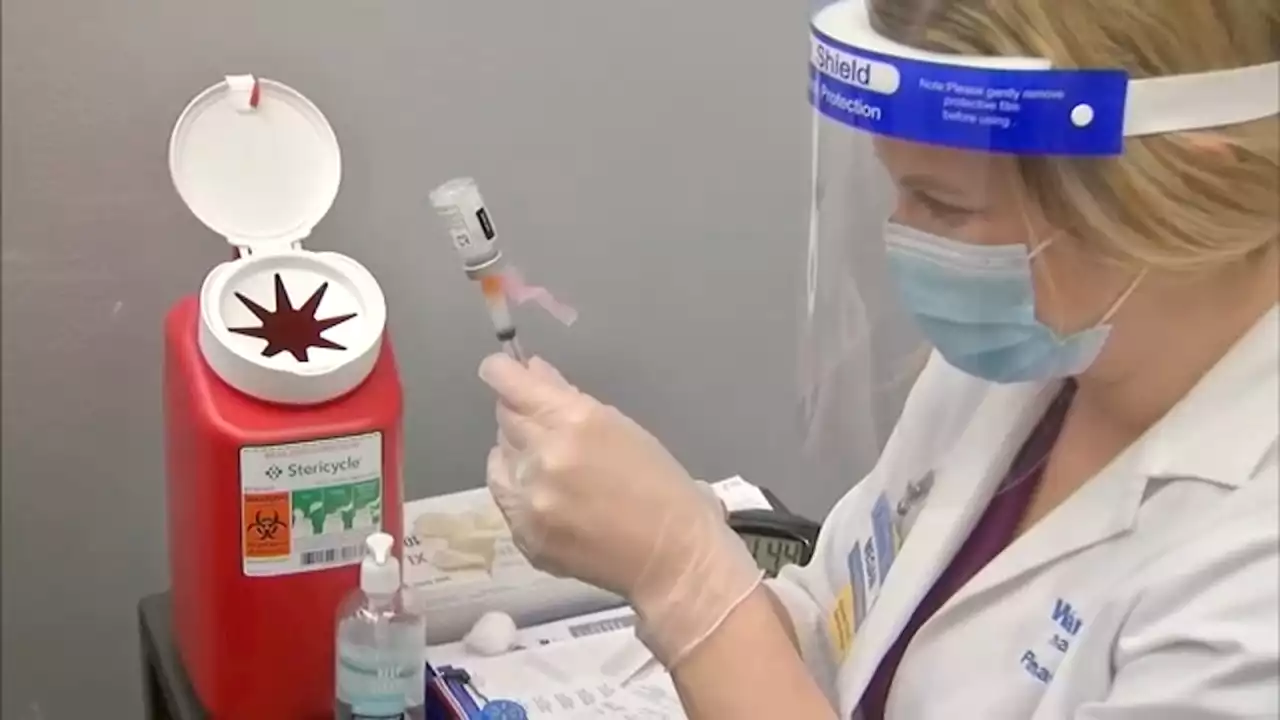 'COVID is not done with us': Why is the BA.5 COVID-19 variant so contagious?The COVID-19 pandemic is far from over, according to experts.
'COVID is not done with us': Why is the BA.5 COVID-19 variant so contagious?The COVID-19 pandemic is far from over, according to experts.
Baca lebih lajut »
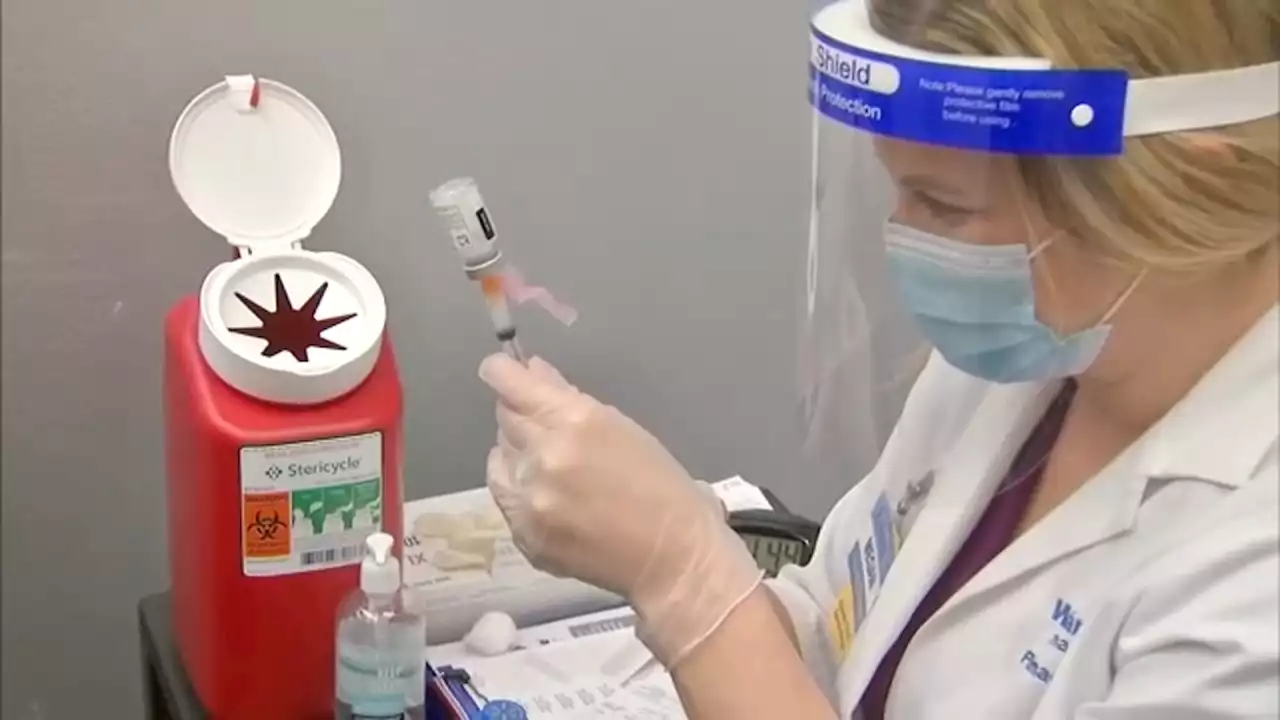 'COVID is not done with us': Why is the BA.5 COVID-19 variant so contagious?The COVID-19 pandemic is far from over, according to experts.
'COVID is not done with us': Why is the BA.5 COVID-19 variant so contagious?The COVID-19 pandemic is far from over, according to experts.
Baca lebih lajut »
 US regulators OK new COVID-19 shot option from NovavaxThe U.S. is getting another COVID-19 vaccine choice
US regulators OK new COVID-19 shot option from NovavaxThe U.S. is getting another COVID-19 vaccine choice
Baca lebih lajut »
 US regulators OK new COVID-19 shot option from NovavaxNovavax makes a more traditional type of shot than the three other COVID-19 vaccines available for use in the U.S.
US regulators OK new COVID-19 shot option from NovavaxNovavax makes a more traditional type of shot than the three other COVID-19 vaccines available for use in the U.S.
Baca lebih lajut »
 U.S. health officials approve new COVID-19 shot from NovavaxThe U.S. is getting another COVID-19 vaccine choice as the Food and Drug Administration on Wednesday cleared Novavax shots for adults. Novavax makes a more...
U.S. health officials approve new COVID-19 shot from NovavaxThe U.S. is getting another COVID-19 vaccine choice as the Food and Drug Administration on Wednesday cleared Novavax shots for adults. Novavax makes a more...
Baca lebih lajut »
