The FDA has cleared for use the Pfizer and Moderna Covid-19 vaccines in children as young as six months. They could start getting the shots as soon as Monday.
Children under five in the U.S. aren’t yet eligible for a Covid-19 vaccine after efforts to get the shots to market have hit setbacks.
WSJ’s Peter Loftus explains what we know about the vaccines and when they might become available. Photo composite: Todd Johnsonfrom Pfizer Inc. and BioNTech SE and from Moderna Inc. in children as young as 6 months.
Indonesia Berita Terbaru, Indonesia Berita utama
Similar News:Anda juga dapat membaca berita serupa dengan ini yang kami kumpulkan dari sumber berita lain.
 FDA Advisers Review Moderna, Pfizer COVID-19 Shots for Kids as Young as 6 monthsU.S. government advisers met Wednesday to decide whether to endorse COVID-19 shots for babies, toddlers and preschoolers, moving the nation closer to vaccinations for all ages.
FDA Advisers Review Moderna, Pfizer COVID-19 Shots for Kids as Young as 6 monthsU.S. government advisers met Wednesday to decide whether to endorse COVID-19 shots for babies, toddlers and preschoolers, moving the nation closer to vaccinations for all ages.
Baca lebih lajut »
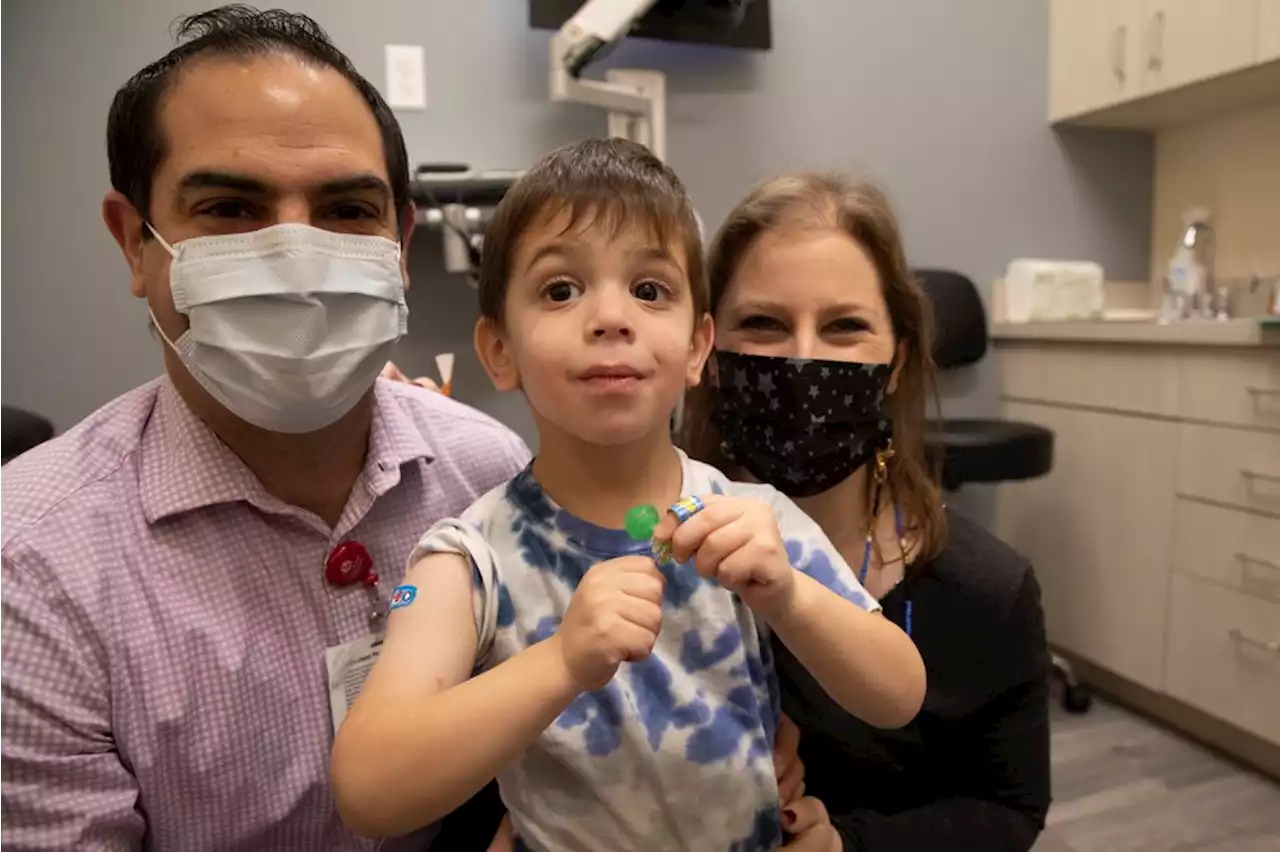 FDA advisers weigh COVID-19 shots for babies, young childrenU.S. government advisers met Wednesday to decide whether to endorse COVID-19 shots for the youngest children, moving the nation closer to vaccinations for all ages.
FDA advisers weigh COVID-19 shots for babies, young childrenU.S. government advisers met Wednesday to decide whether to endorse COVID-19 shots for the youngest children, moving the nation closer to vaccinations for all ages.
Baca lebih lajut »
 FDA Advisers Move Moderna's COVID-19 Shots Closer To Kids Under 5The outside experts voted unanimously that the benefits of Moderna’s shots outweigh any risks for children under 5.
FDA Advisers Move Moderna's COVID-19 Shots Closer To Kids Under 5The outside experts voted unanimously that the benefits of Moderna’s shots outweigh any risks for children under 5.
Baca lebih lajut »
 FDA panel says Pfizer, Moderna's COVID-19 shots for tots safe and effectiveUPDATE: FDA panel says Pfizer, Moderna's COVID-19 shots for kids under 5 are safe and effective
FDA panel says Pfizer, Moderna's COVID-19 shots for tots safe and effectiveUPDATE: FDA panel says Pfizer, Moderna's COVID-19 shots for kids under 5 are safe and effective
Baca lebih lajut »
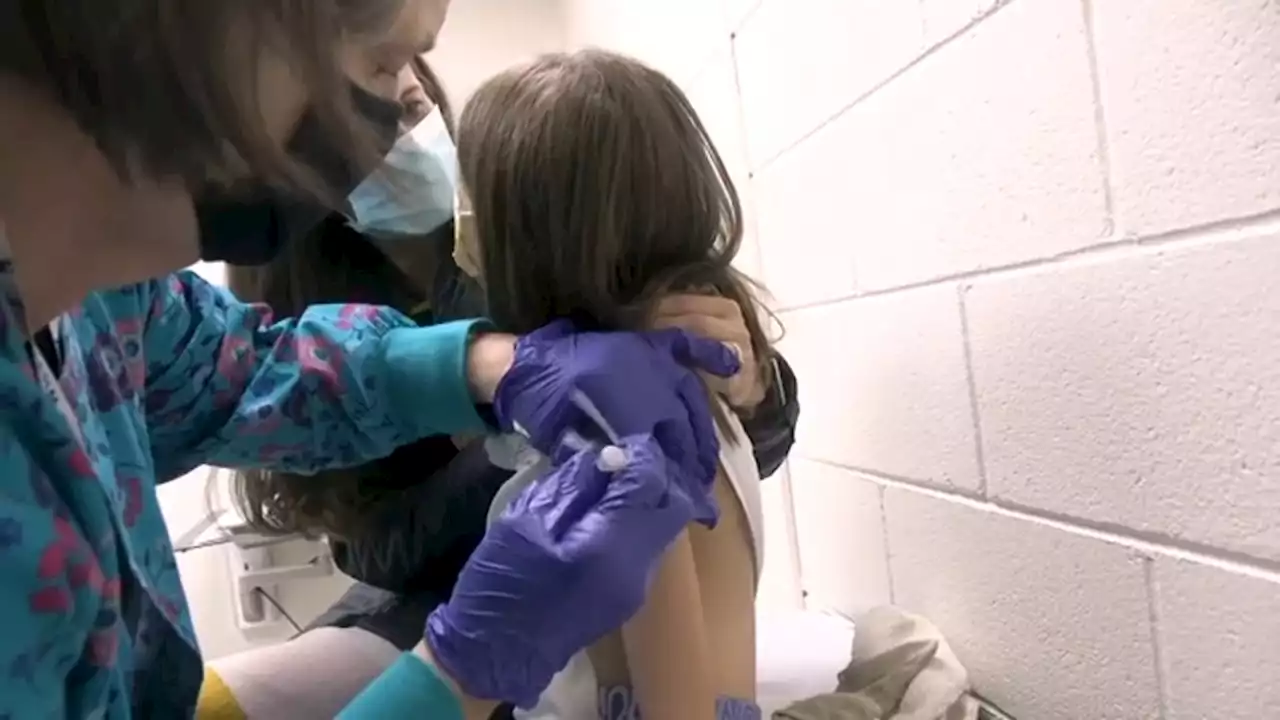 FDA advisory panel backs COVID-19 vaccine for younger kids, the last unprotected groupIf all the regulatory steps are cleared, shots should be available next week.
FDA advisory panel backs COVID-19 vaccine for younger kids, the last unprotected groupIf all the regulatory steps are cleared, shots should be available next week.
Baca lebih lajut »
 FDA advisers endorse emergency use of Moderna, Pfizer Covid-19 vaccine in babies, toddlersFDA advisers voted unanimously to endorse Moderna's Covid-19 vaccines for babies and toddlers. The FDA is expected to quickly authorize the vaccine for emergency use. A vote on Pfizer's vaccine is coming soon.
FDA advisers endorse emergency use of Moderna, Pfizer Covid-19 vaccine in babies, toddlersFDA advisers voted unanimously to endorse Moderna's Covid-19 vaccines for babies and toddlers. The FDA is expected to quickly authorize the vaccine for emergency use. A vote on Pfizer's vaccine is coming soon.
Baca lebih lajut »
