While at-home COVID tests are readily available, this is the first home test for influenza A and B, commonly known as the flu.
The test was granted an emergency use authorization, which facilitates the availability of “medical countermeasures” during public health emergencies.
Jeff Shuren, director of the FDA’s Center for Devices and Radiological Health, called the authorization “a major milestone in bringing greater consumer access to diagnostic tests that can be performed entirely at home.” The agency said the test is for individuals “with signs and symptoms consistent with a respiratory tract infection” and said it can be used on children as young as 2, with adults collecting the samples.
It recommends that tests be reported to healthcare providers and cautions that there is a risk of false positive and negative results. “Individuals who test negative and continue to experience symptoms of fever, cough and-or shortness of breath may still have a respiratory infection and should seek follow-up care with their healthcare provider,” the agency said.
Citing the impact of COVID and RSV, another respiratory infection, the FDA said it “recognizes the benefits that home testing can provide” and would work to increase the number of tests available.
Indonesia Berita Terbaru, Indonesia Berita utama
Similar News:Anda juga dapat membaca berita serupa dengan ini yang kami kumpulkan dari sumber berita lain.
 FDA approves combination flu-COVID test for home useThis is the first home test for the flu.
FDA approves combination flu-COVID test for home useThis is the first home test for the flu.
Baca lebih lajut »
 FDA approves first at-home test to detect both flu and COVIDThe FDA has authorized the first over-the-counter home test that can detect both influenza A and B as well as SARS-CoV-2, the virus that causes COVID-19.
FDA approves first at-home test to detect both flu and COVIDThe FDA has authorized the first over-the-counter home test that can detect both influenza A and B as well as SARS-CoV-2, the virus that causes COVID-19.
Baca lebih lajut »
 FDA Authorizes First At-Home Combo Test for COVID and FluThe Food and Drug Administration has granted emergency use authorization for the first over-the-counter, at-home test that detects both COVID-19 and influenza, the agency announced Friday.
FDA Authorizes First At-Home Combo Test for COVID and FluThe Food and Drug Administration has granted emergency use authorization for the first over-the-counter, at-home test that detects both COVID-19 and influenza, the agency announced Friday.
Baca lebih lajut »
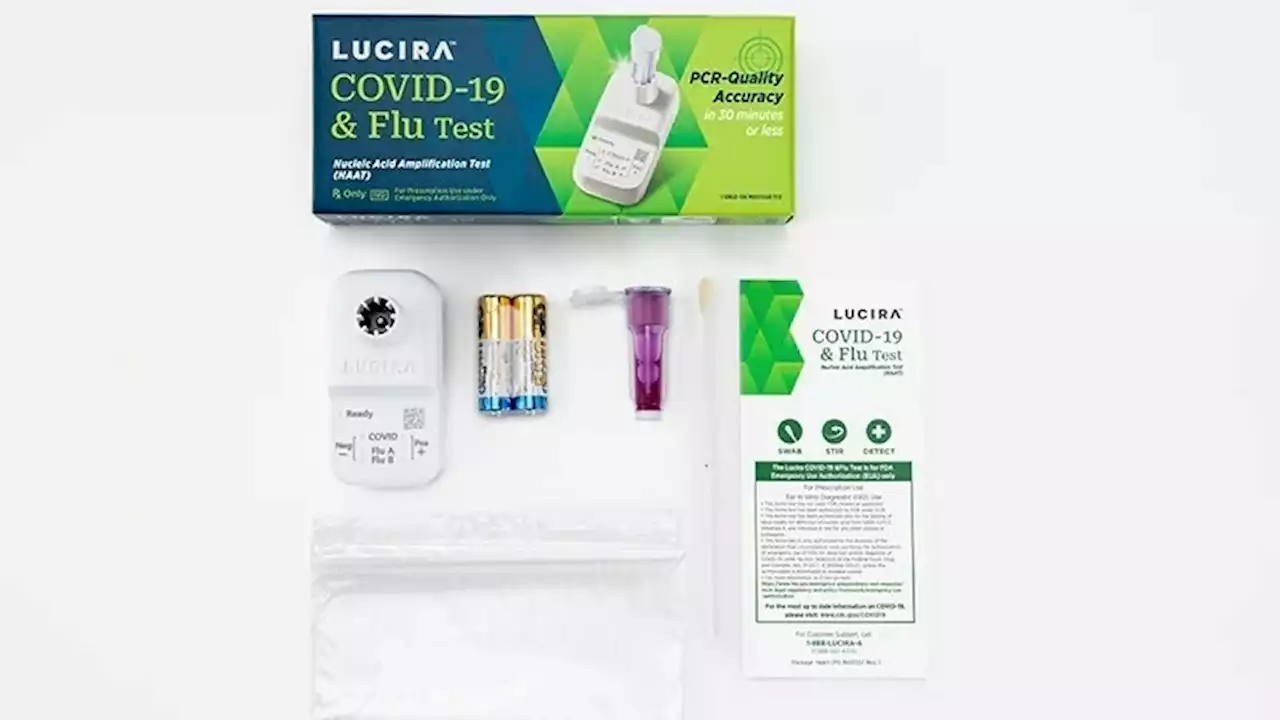 FDA authorizes the first at-home test for COVID-19 and the fluThe FDA has authorized the first at-home test that can simultaneously detect both COVID and the flu. The nasal swab provides results within 30 minutes.
FDA authorizes the first at-home test for COVID-19 and the fluThe FDA has authorized the first at-home test that can simultaneously detect both COVID and the flu. The nasal swab provides results within 30 minutes.
Baca lebih lajut »
 PolitiFact - FDA approval is required for COVID-19 vaccine manufacturers to change ingredients in shotsThe COVID-19 vaccines from Pfizer and Moderna are no longer under an emergency use authorization for adults. Even if they were, any changes would require the manufacturers to submit data and have their product reassessed by the FDA.
PolitiFact - FDA approval is required for COVID-19 vaccine manufacturers to change ingredients in shotsThe COVID-19 vaccines from Pfizer and Moderna are no longer under an emergency use authorization for adults. Even if they were, any changes would require the manufacturers to submit data and have their product reassessed by the FDA.
Baca lebih lajut »
