U.S. regulators on Friday authorized the first COVID-19 shots for infants and preschoolers, paving the way for vaccinations to begin next week.
The Food and Drug Administration’s action follows its advisory panel’s unanimous recommendation for the shots from Moderna and Pfizer. That means U.S. kids under 5 — roughly 18 million youngsters — are eligible for the shots, about 1 1/2 years after the vaccines first became available in the U.S. for adults, who have been hit the hardest during the pandemic.
She said pediatric deaths from COVID-19 have been higher than what is generally seen from the flu each year. For weeks, the Biden administration has been preparing to roll out the vaccines for little kids, with states, tribes, community health centers and pharmacies preordering millions of doses. FDA’s emergency use authorization allows manufacturers to begin shipping vaccine across the country. Vaccinations could begin early next week.
In testing, the littlest children developed high levels of virus-fighting antibodies, comparable to what is seen in young adults, the FDA said. Moderna’s vaccine was about 40% to 50% effective at preventing infections but there were too few cases during Pfizer’s study to reliably determine effectiveness, the agency said.
Both vaccines are for children as young as 6 months. Moderna next plans to study its shots for babies as young as 3 months. Pfizer has not finalized plans for shots in younger infants. A dozen countries, including China, already vaccinate kids under 5, with other brands.
Indonesia Berita Terbaru, Indonesia Berita utama
Similar News:Anda juga dapat membaca berita serupa dengan ini yang kami kumpulkan dari sumber berita lain.
 COVID-19 vaccine: FDA authorizes 1st shots for kids under 5; CDC review nextBREAKING: U.S. regulators on Friday authorized the first COVID-19 shots for infants and preschoolers, paving the way for vaccinations to begin next week.
COVID-19 vaccine: FDA authorizes 1st shots for kids under 5; CDC review nextBREAKING: U.S. regulators on Friday authorized the first COVID-19 shots for infants and preschoolers, paving the way for vaccinations to begin next week.
Baca lebih lajut »
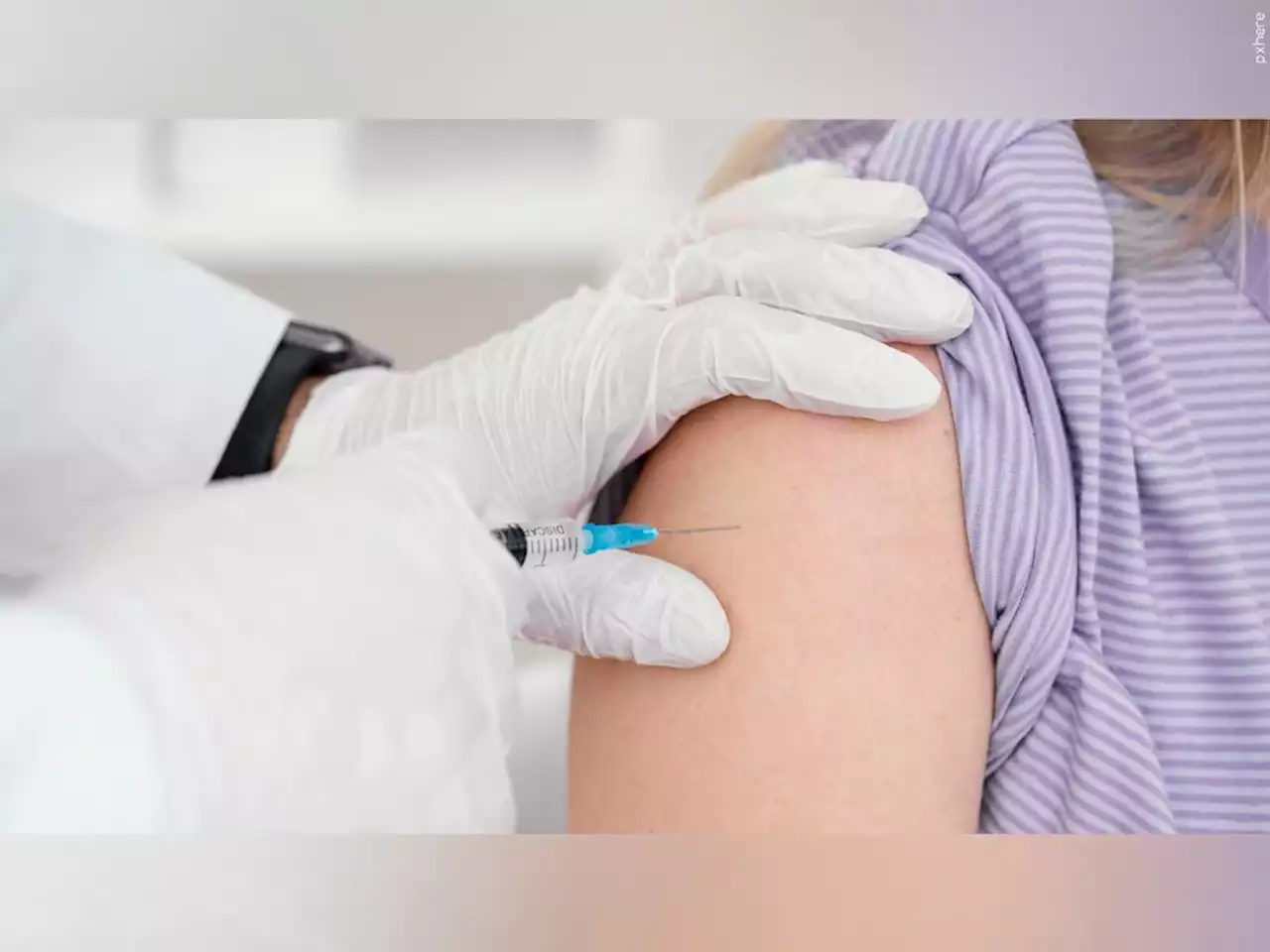 FDA authorizes 1st COVID-19 shots for infants, preschoolersFDA authorizes 1st COVID-19 shots for infants, preschoolers.
FDA authorizes 1st COVID-19 shots for infants, preschoolersFDA authorizes 1st COVID-19 shots for infants, preschoolers.
Baca lebih lajut »
 FDA Authorizes 1st COVID-19 Shots for Infants, PreschoolersJUST IN: The FDA has authorized the first COVID-19 shots for children under the age of five, paving the way for vaccinations to begin once CDC approves.
FDA Authorizes 1st COVID-19 Shots for Infants, PreschoolersJUST IN: The FDA has authorized the first COVID-19 shots for children under the age of five, paving the way for vaccinations to begin once CDC approves.
Baca lebih lajut »
 FDA authorizes 1st COVID-19 shots for kids under 5; CDC review is nextU.S. regulators on Friday authorized the first COVID-19 shots for infants and preschoolers, paving the way for vaccinations to begin next week.
FDA authorizes 1st COVID-19 shots for kids under 5; CDC review is nextU.S. regulators on Friday authorized the first COVID-19 shots for infants and preschoolers, paving the way for vaccinations to begin next week.
Baca lebih lajut »
 FDA authorizes 1st COVID-19 shots for infants, preschoolersU.S. regulators have authorized the first COVID-19 shots for infants and preschoolers.
FDA authorizes 1st COVID-19 shots for infants, preschoolersU.S. regulators have authorized the first COVID-19 shots for infants and preschoolers.
Baca lebih lajut »
 FDA advisers endorse 1st COVID-19 shots for kids under 5The first COVID-19 shots for infants, toddlers and preschoolers in the U.S. have moved a step closer
FDA advisers endorse 1st COVID-19 shots for kids under 5The first COVID-19 shots for infants, toddlers and preschoolers in the U.S. have moved a step closer
Baca lebih lajut »