JUST IN: The FDA has authorized the first COVID-19 shots for children under the age of five, paving the way for vaccinations to begin once CDC approves.
For weeks, the Biden administration has been preparing to roll out the vaccines. States, tribes, community health centers and pharmacies preordered millions of doses. FDA's emergency use authorization allows manufacturers to begin shipping vaccine across the country. Vaccinations could begin as early as Monday or Tuesday.
Moderna's is two shots, each a quarter of its adult dose, given about four weeks apart for kids under 6. The vaccines are for children as young as 6 months. Moderna next plans to study its shots for babies as young as 3-months-old. Pfizer has not finalized plans for shots in younger infants. A dozen countries, including China, already vaccinate kids under 5.
Dr. Beth Ebel, professor of pediatrics at University of Washington in Seattle, said the tot-sized vaccines would be especially welcomed by U.S. parents with children in daycare where outbreaks can sideline parents from jobs, adding to financial strain. “A lot of people are going to be happy and a lot of grandparents are going to be happy, too, because we’ve missed those babies who grew up when you weren’t able to see them,” Ebel said.Follow AP Medical Writer Lindsey Tanner at @LindseyTanner.The Associated Press Health and Science Department receives support from the Howard Hughes Medical Institute’s Department of Science Education. The AP is solely responsible for all content.
Indonesia Berita Terbaru, Indonesia Berita utama
Similar News:Anda juga dapat membaca berita serupa dengan ini yang kami kumpulkan dari sumber berita lain.
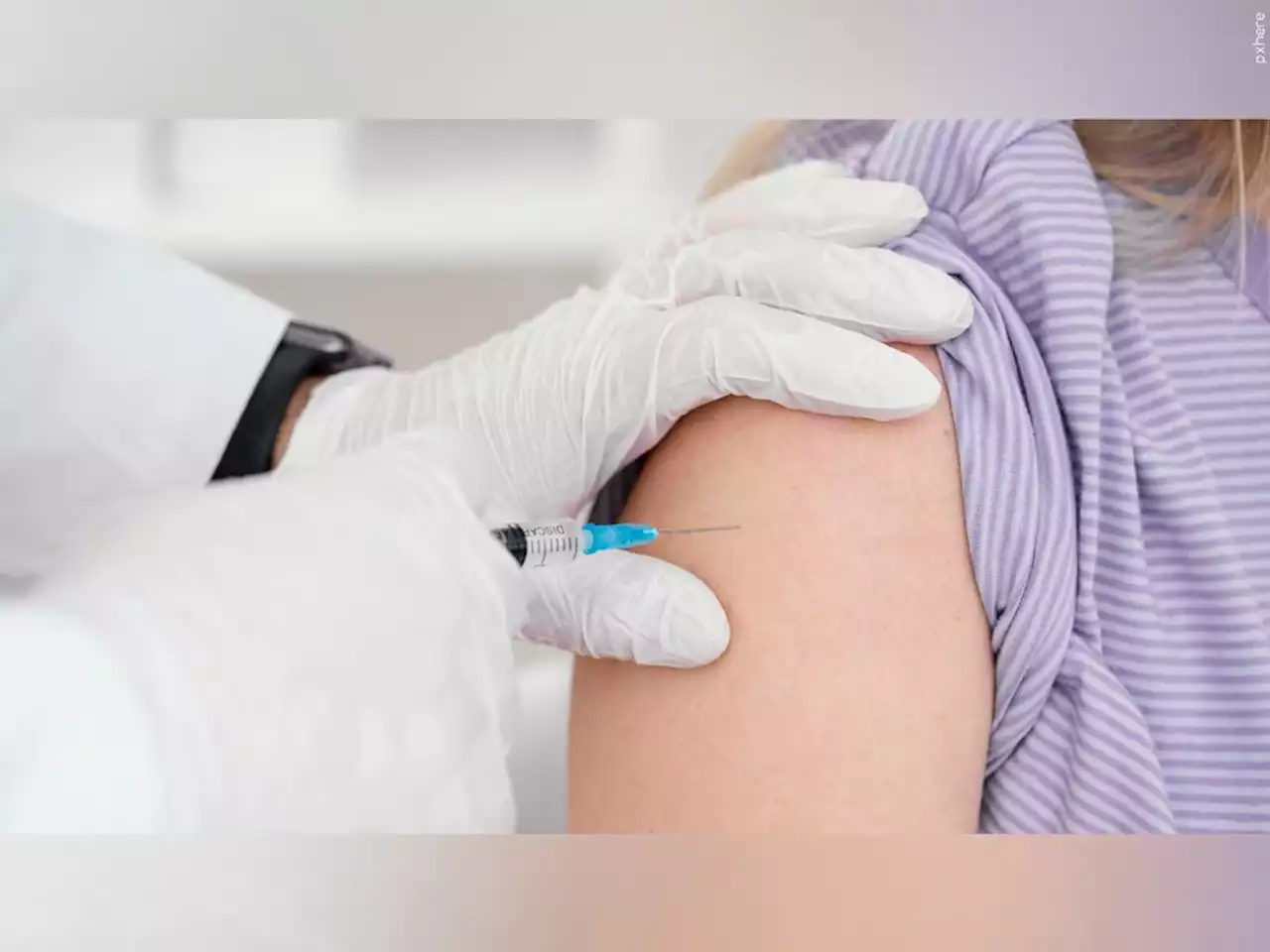 FDA authorizes 1st COVID-19 shots for infants, preschoolersFDA authorizes 1st COVID-19 shots for infants, preschoolers.
FDA authorizes 1st COVID-19 shots for infants, preschoolersFDA authorizes 1st COVID-19 shots for infants, preschoolers.
Baca lebih lajut »
 FDA Advisers Review Moderna, Pfizer COVID-19 Shots for Kids as Young as 6 monthsU.S. government advisers met Wednesday to decide whether to endorse COVID-19 shots for babies, toddlers and preschoolers, moving the nation closer to vaccinations for all ages.
FDA Advisers Review Moderna, Pfizer COVID-19 Shots for Kids as Young as 6 monthsU.S. government advisers met Wednesday to decide whether to endorse COVID-19 shots for babies, toddlers and preschoolers, moving the nation closer to vaccinations for all ages.
Baca lebih lajut »
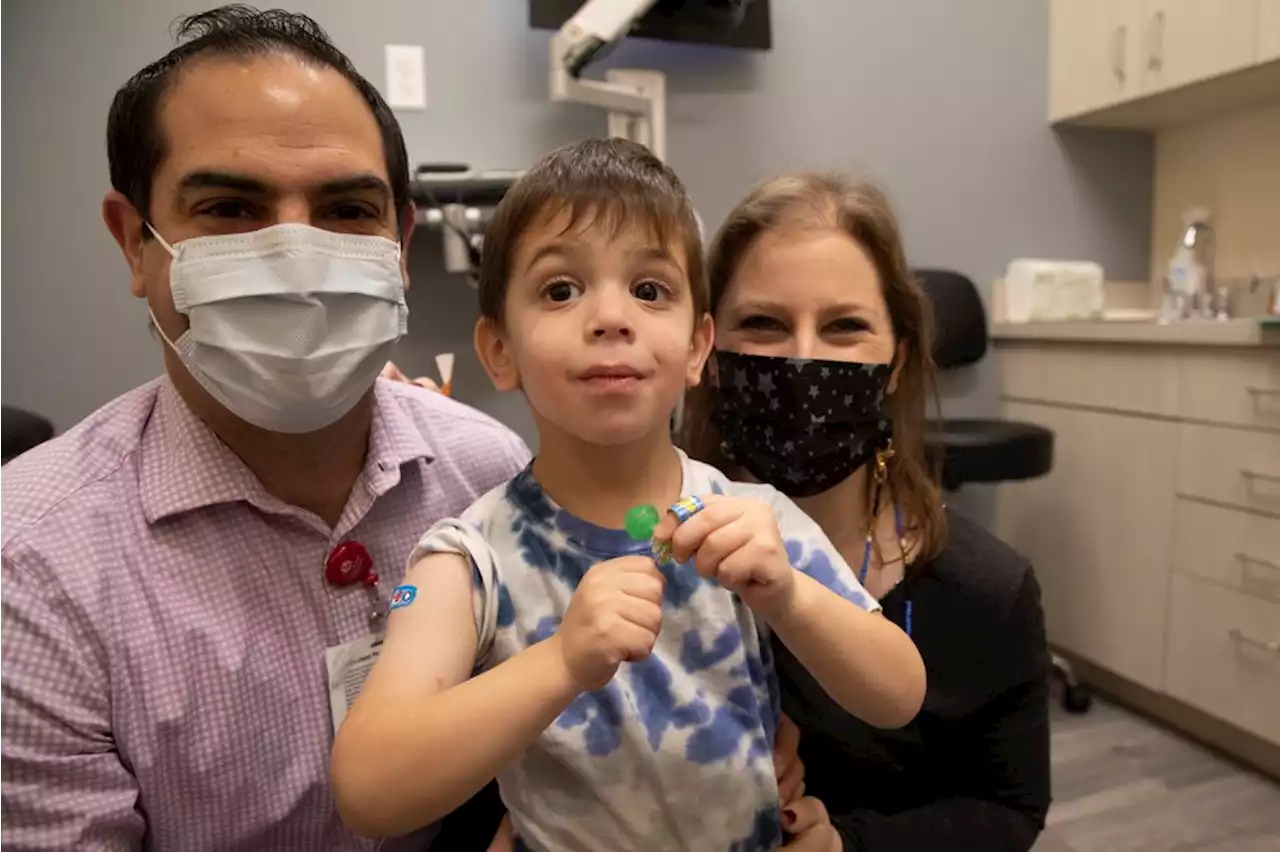 FDA advisers weigh COVID-19 shots for babies, young childrenU.S. government advisers met Wednesday to decide whether to endorse COVID-19 shots for the youngest children, moving the nation closer to vaccinations for all ages.
FDA advisers weigh COVID-19 shots for babies, young childrenU.S. government advisers met Wednesday to decide whether to endorse COVID-19 shots for the youngest children, moving the nation closer to vaccinations for all ages.
Baca lebih lajut »
 FDA Advisers Move Moderna's COVID-19 Shots Closer To Kids Under 5The outside experts voted unanimously that the benefits of Moderna’s shots outweigh any risks for children under 5.
FDA Advisers Move Moderna's COVID-19 Shots Closer To Kids Under 5The outside experts voted unanimously that the benefits of Moderna’s shots outweigh any risks for children under 5.
Baca lebih lajut »
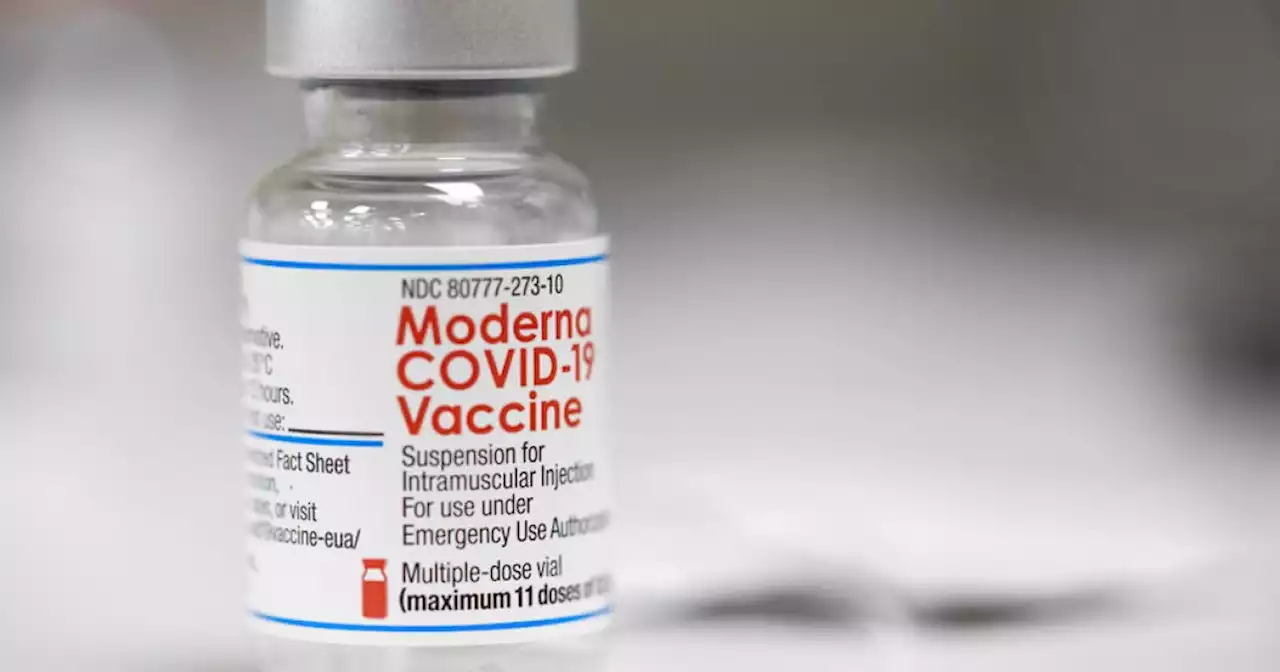 FDA panel says Pfizer, Moderna's COVID-19 shots for tots safe and effectiveUPDATE: FDA panel says Pfizer, Moderna's COVID-19 shots for kids under 5 are safe and effective
FDA panel says Pfizer, Moderna's COVID-19 shots for tots safe and effectiveUPDATE: FDA panel says Pfizer, Moderna's COVID-19 shots for kids under 5 are safe and effective
Baca lebih lajut »
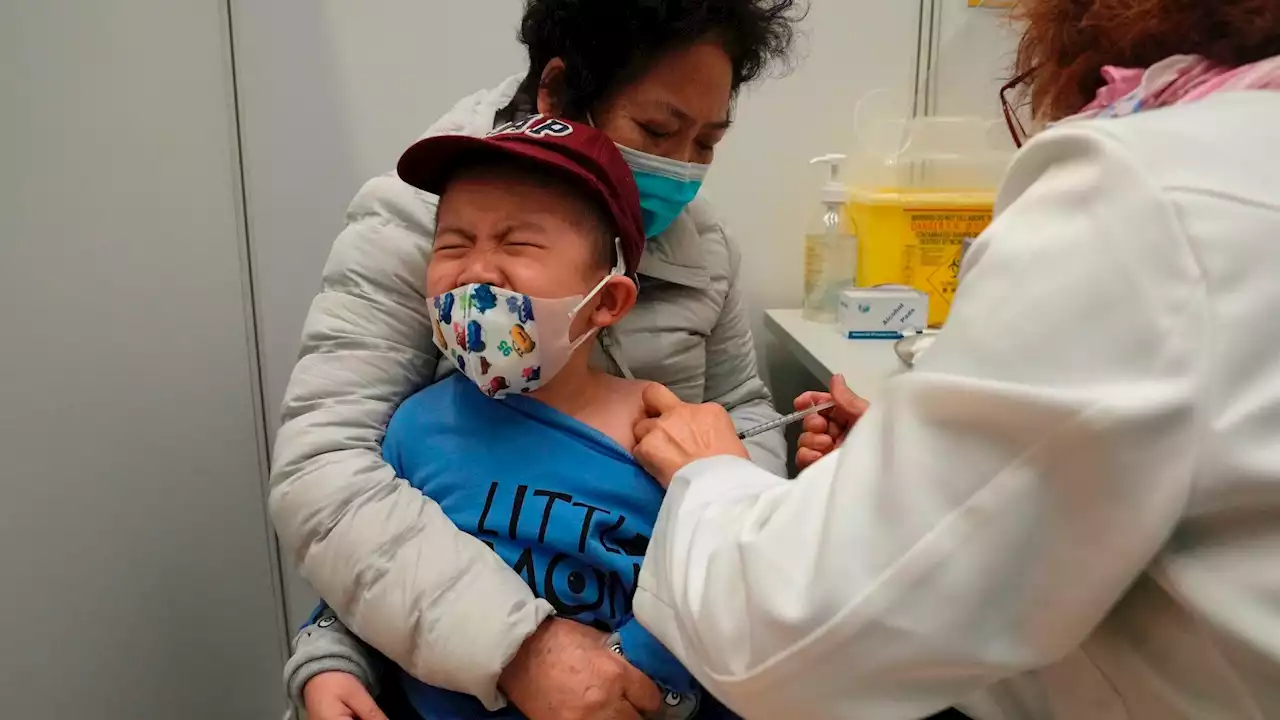 FDA Advisers Move COVID-19 Shots Closer for Kids Under 5The Food and Drug Administration’s vaccine advisers gave a thumbs-up to vaccines from Moderna and Pfizer for the littlest kids.
FDA Advisers Move COVID-19 Shots Closer for Kids Under 5The Food and Drug Administration’s vaccine advisers gave a thumbs-up to vaccines from Moderna and Pfizer for the littlest kids.
Baca lebih lajut »
