If authorized by the FDA, it would be just the second Covid vaccine available to teens and schoolchildren in the U.S.
. That’s mostly a risk for teen boys and young men, and also can occur with the Pfizer vaccine. Moderna got extra scrutiny because its shots are a far higher dose., FDA scientists said there were no confirmed cases of the heart inflammation in Moderna’s kid studies. But experts say the studies may have had too few participants for a rare side effect like that to appear.
As for other side effects, FDA officials said nothing worrisome was reported — mainly sore arms, headache and fatigue.
Indonesia Berita Terbaru, Indonesia Berita utama
Similar News:Anda juga dapat membaca berita serupa dengan ini yang kami kumpulkan dari sumber berita lain.
 Dogs Can Sniff Out COVID-19 and Signs of Long COVID, Studies SuggestAlmost any dog can be trained to do this type of detection in a matter of weeks, researchers say
Dogs Can Sniff Out COVID-19 and Signs of Long COVID, Studies SuggestAlmost any dog can be trained to do this type of detection in a matter of weeks, researchers say
Baca lebih lajut »
 FDA advisers back Moderna's COVID-19 vaccine for older kidsA government advisory panel has endorsed a second brand of COVID-19 vaccine for school-age children and teens.
FDA advisers back Moderna's COVID-19 vaccine for older kidsA government advisory panel has endorsed a second brand of COVID-19 vaccine for school-age children and teens.
Baca lebih lajut »
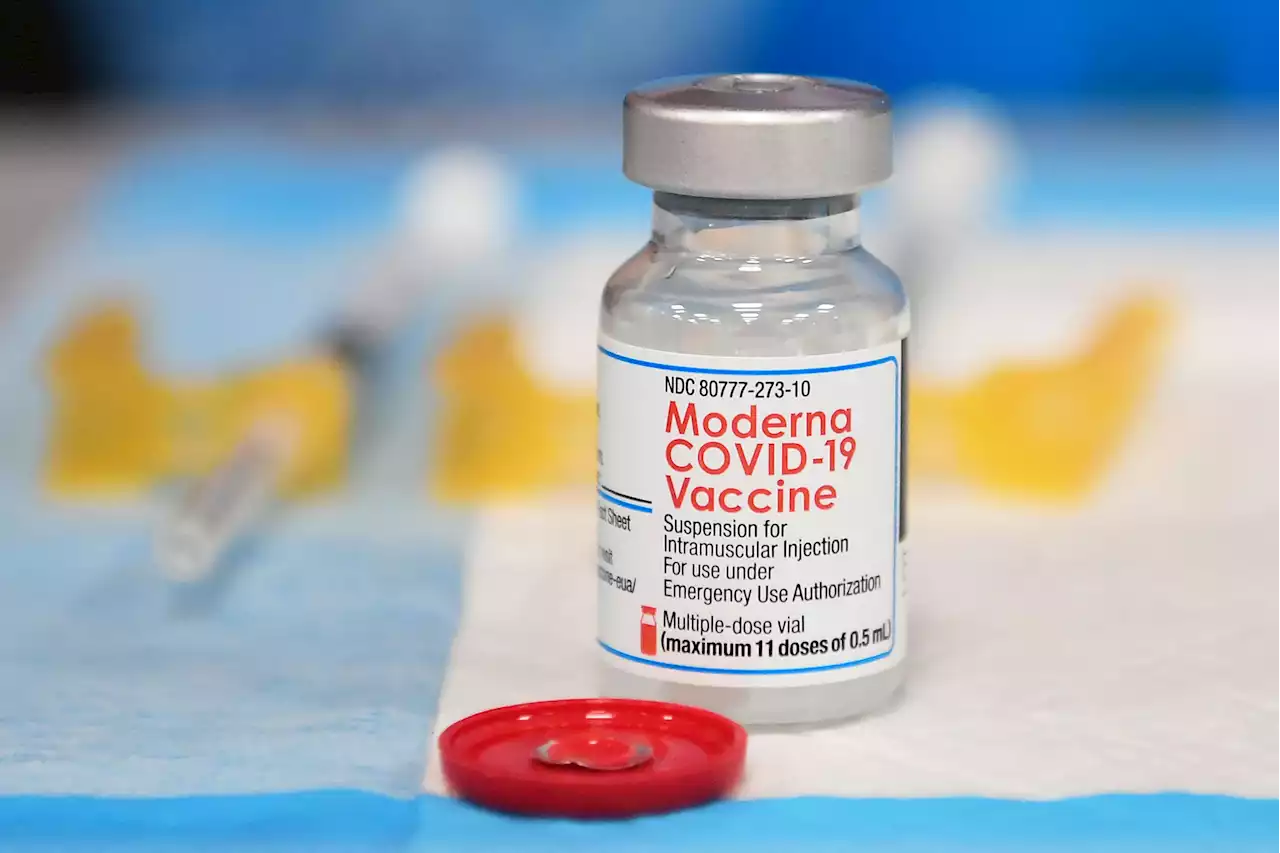 FDA Advisers Back Moderna's COVID-19 Vaccine for Older KidsA government advisory panel Tuesday endorsed a second brand of COVID-19 vaccine for school-age children and teens. The Food and Drug Administration’s outside experts voted unanimously that Moderna’s vaccine is safe and effective enough to give kids ages 6 to 17. If the FDA agrees, it would become the second option for those children, joining Pfizer’s vaccine. The same FDA…
FDA Advisers Back Moderna's COVID-19 Vaccine for Older KidsA government advisory panel Tuesday endorsed a second brand of COVID-19 vaccine for school-age children and teens. The Food and Drug Administration’s outside experts voted unanimously that Moderna’s vaccine is safe and effective enough to give kids ages 6 to 17. If the FDA agrees, it would become the second option for those children, joining Pfizer’s vaccine. The same FDA…
Baca lebih lajut »
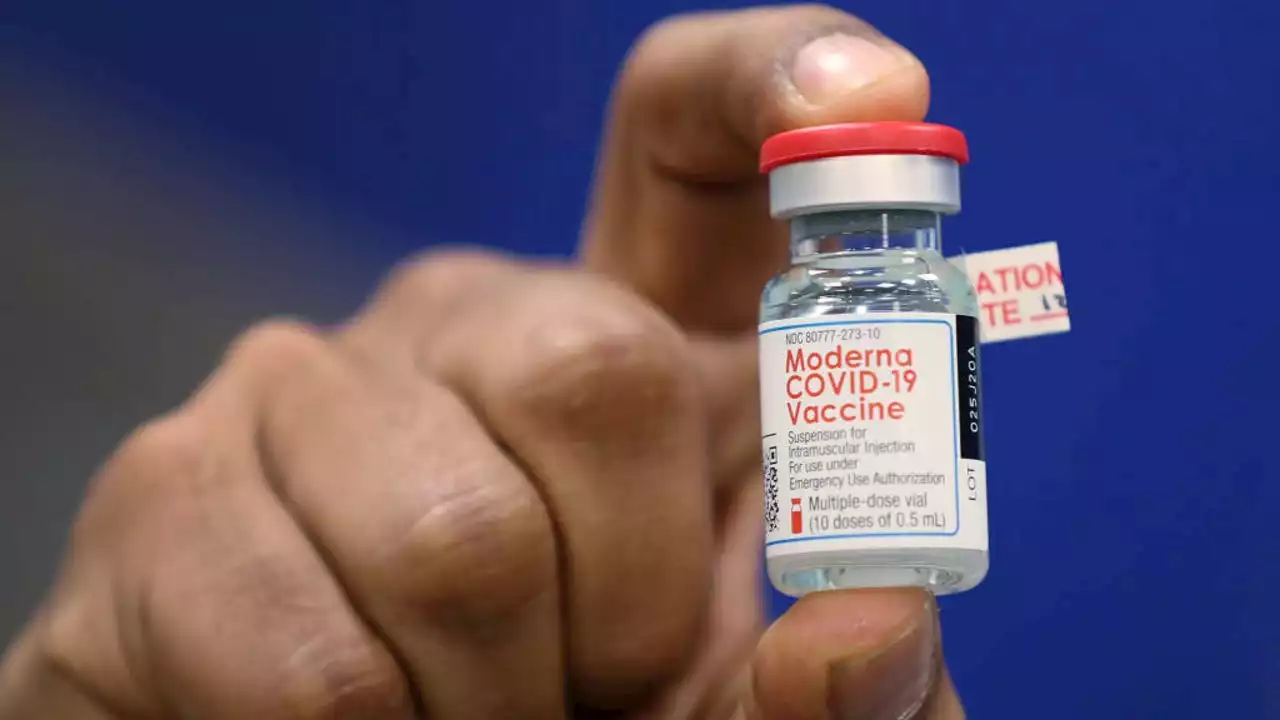 FDA panel endorses Moderna's COVID vaccine for teens, school-age kidsAn FDA advisory panel endorsed on Tuesday Moderna's COVID-19 vaccine for teenagers and school-aged children.
FDA panel endorses Moderna's COVID vaccine for teens, school-age kidsAn FDA advisory panel endorsed on Tuesday Moderna's COVID-19 vaccine for teenagers and school-aged children.
Baca lebih lajut »
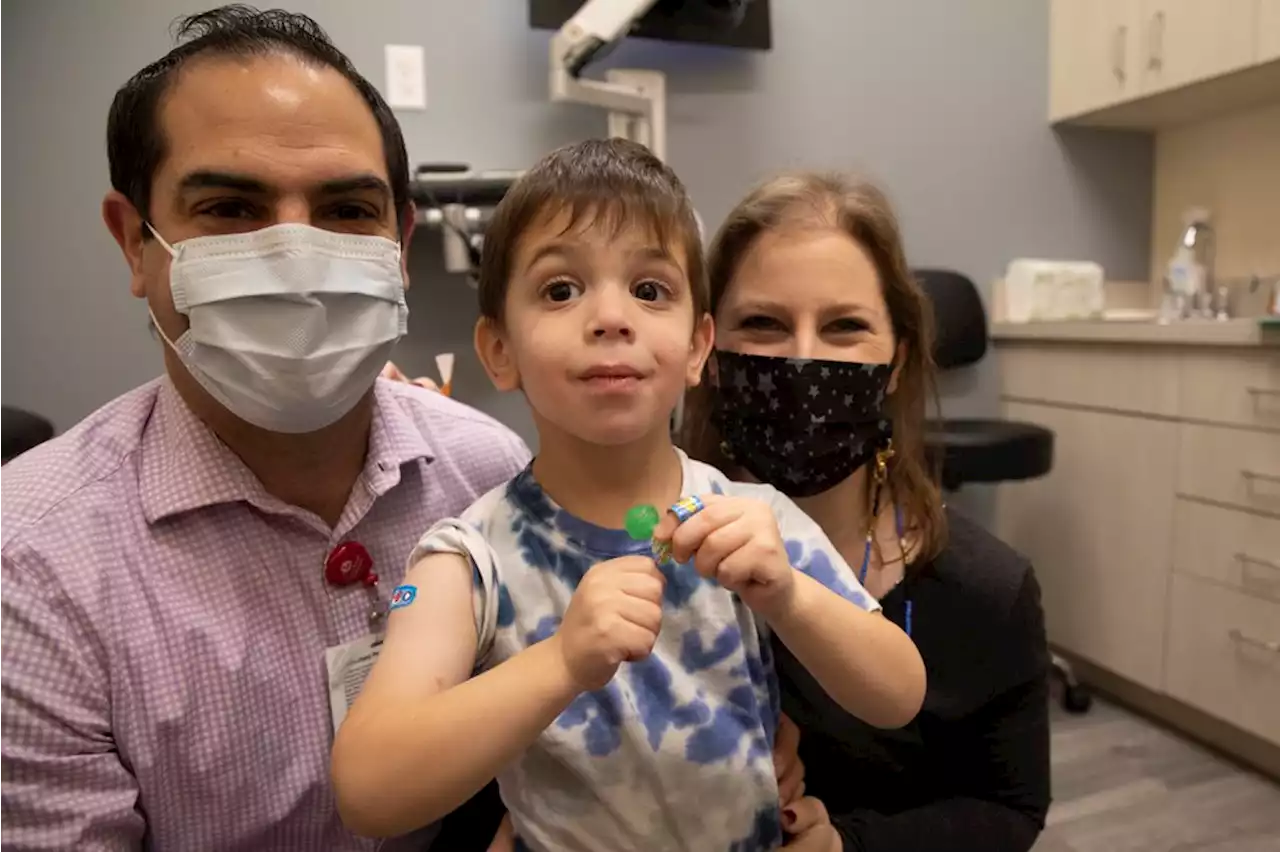 FDA advisers back Moderna’s COVID-19 vaccine for teens, younger kidsA government advisory panel Tuesday endorsed a second brand of COVID-19 vaccine for school-age children and teens.
FDA advisers back Moderna’s COVID-19 vaccine for teens, younger kidsA government advisory panel Tuesday endorsed a second brand of COVID-19 vaccine for school-age children and teens.
Baca lebih lajut »
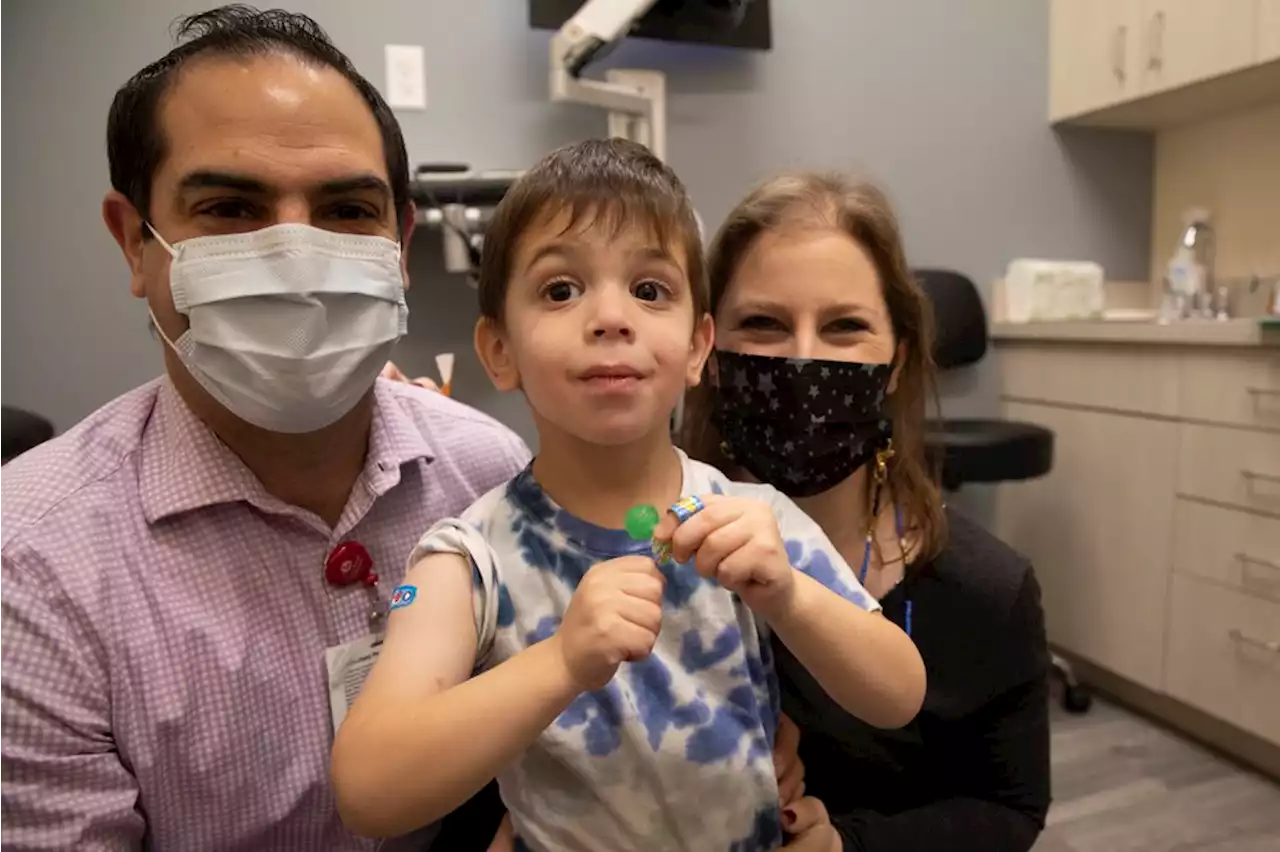 FDA advisers consider Moderna’s COVID shots for teens, younger kidsFDA scientists said there were no confirmed cases of the heart inflammation in Moderna’s kid studies.
FDA advisers consider Moderna’s COVID shots for teens, younger kidsFDA scientists said there were no confirmed cases of the heart inflammation in Moderna’s kid studies.
Baca lebih lajut »
