An expert panel backed a second COVID-19 vaccine option for kids ages 6 to 17 Thursday.
–
The panel’s recommendations usually are adopted by the CDC, and become the government’s guidance for U.S. doctors and their patients. The FDA also authorized a third dose for kids with significantly weakened immune systems, to be given about a month after the second dose of the primary series. The CDC is expected to recommend the same thing.How much demand there will be for the shots isn’t clear. Teens became eligible a year ago for Pfizer’s vaccine, which uses the same technology, and only 60% have gotten two doses. Shots for younger kids started in November; about 29% have been fully vaccinated, according to the CDC.
Indonesia Berita Terbaru, Indonesia Berita utama
Similar News:Anda juga dapat membaca berita serupa dengan ini yang kami kumpulkan dari sumber berita lain.
 CDC panel recommends Moderna two-dose Covid vaccine for kids ages 6 to 17CDC Director Dr. Rochelle Walensky is expected to sign off on the recommendation later Thursday,.
CDC panel recommends Moderna two-dose Covid vaccine for kids ages 6 to 17CDC Director Dr. Rochelle Walensky is expected to sign off on the recommendation later Thursday,.
Baca lebih lajut »
 Biden visits COVID-19 vaccine clinic as kids 6 months and up receive their 1st shotThe nation’s youngest children are getting their chance at vaccines for COVID-19. Shots began Monday at a few locations, though they were expected to ramp up after the Juneteenth federal holiday.
Biden visits COVID-19 vaccine clinic as kids 6 months and up receive their 1st shotThe nation’s youngest children are getting their chance at vaccines for COVID-19. Shots began Monday at a few locations, though they were expected to ramp up after the Juneteenth federal holiday.
Baca lebih lajut »
 Pa. woman awaits sentencing for making COVID-19 vaccination cardAmy Leister made the card to avoid being quarantined by her place of employment after returning from her daughter's wedding in North Carolina.
Pa. woman awaits sentencing for making COVID-19 vaccination cardAmy Leister made the card to avoid being quarantined by her place of employment after returning from her daughter's wedding in North Carolina.
Baca lebih lajut »
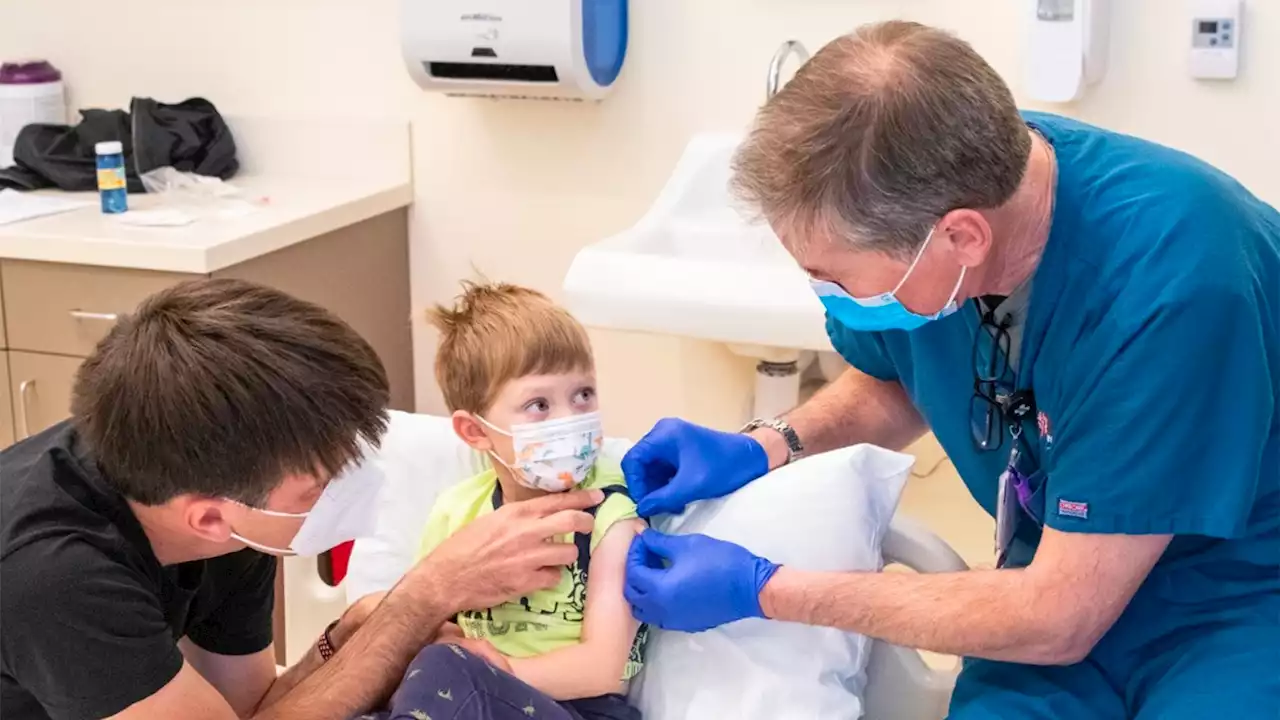 Pfizer’s and Moderna’s COVID-19 vaccines are OK’d for the youngest kids“Having cared for many children that have been in the ICU on ventilators for COVID … and having cared for several children that have died of COVID, we need to be able to prevent COVID-19.”
Pfizer’s and Moderna’s COVID-19 vaccines are OK’d for the youngest kids“Having cared for many children that have been in the ICU on ventilators for COVID … and having cared for several children that have died of COVID, we need to be able to prevent COVID-19.”
Baca lebih lajut »