The FDA granted full approval for adults with COVID-19 who face high risks of severe disease, which can lead to hospitalization or death.
The decision allows Pfizer’s drug to remain on the market indefinitely and to be marketed similarly to other drugs.The U.S. government has stockpiled millions of doses of Paxlovid and patients will continue to receive it at no charge, the FDA said in a statement. More than 14,000 new COVID-19 cases were reported each week last month, although most U.S. cases are no longer reported to health authorities.
Paxlovid is the fourth drug for COVID-19 to receive full FDA approval and the first one that is a pill. The previously approved therapies are Pfizer originally studied Paxlovid in the highest-risk COVID-19 patients: unvaccinated adults with other health problems and no evidence of prior coronavirus infection. In that group, the FDA said the drug lowered the risk of hospitalization or death by 86% when given shortly after symptoms emerged.
But that doesn’t reflect the U.S. population today, where more than 95% of people have protection from at least one vaccine dose, a prior infection or both.In more recent studies of people who have had COVID-19, Paxlovid still significantly decreased the chance of hospitalization or death by more than 85%.
As Paxlovid became widely used in 2021, doctors and patients reported cases of COVID-19 symptoms returning several days after treatment with the drug. But the FDA said Thursday “there is not a clear association,” between Pfizer’s drug and rebound cases., who voted to recommend the drug’s full approval at a meeting earlier this year.
Indonesia Berita Terbaru, Indonesia Berita utama
Similar News:Anda juga dapat membaca berita serupa dengan ini yang kami kumpulkan dari sumber berita lain.
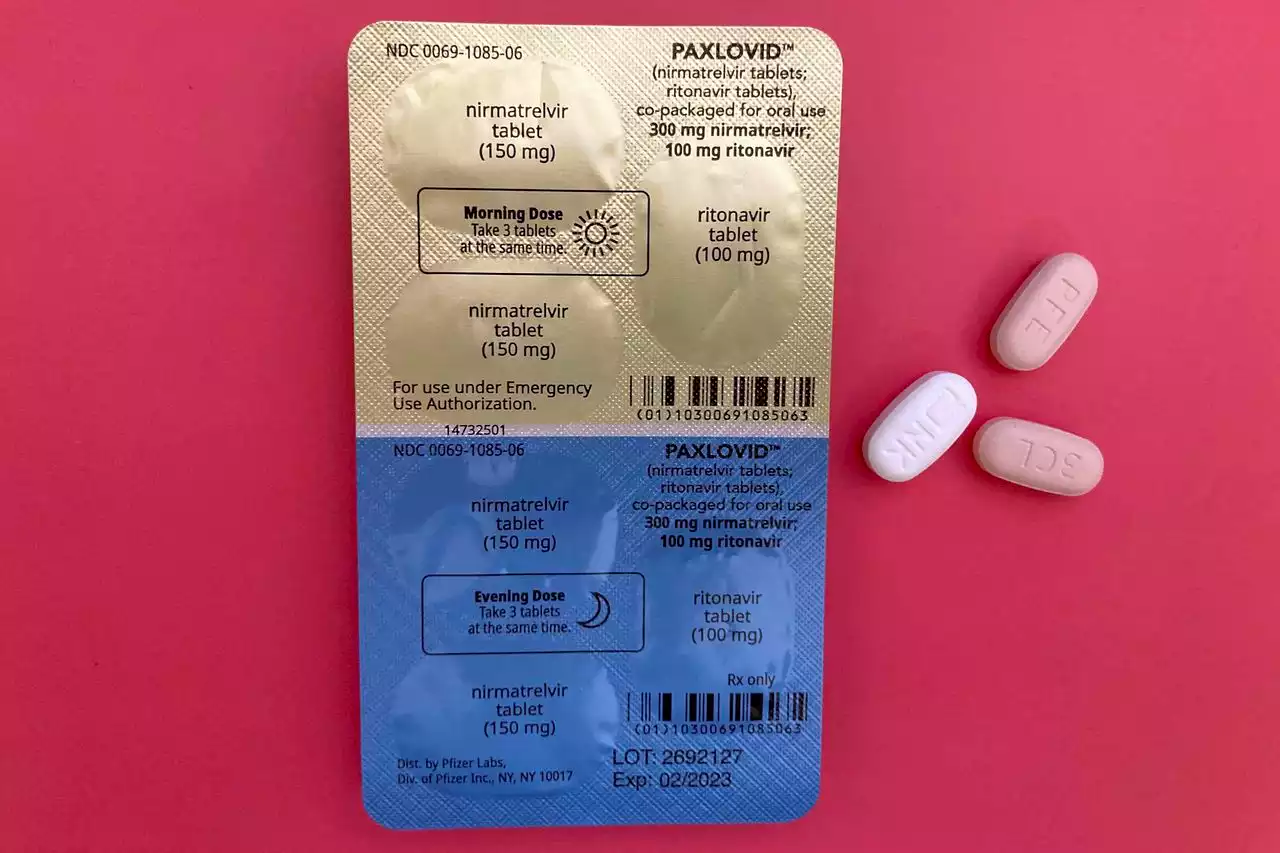 COVID-19 pill Paxlovid gets full FDA approval after over a year of emergency useThursday’s action means the drug has now been fully vetted by the U.S. government and can remain on the market indefinitely.
COVID-19 pill Paxlovid gets full FDA approval after over a year of emergency useThursday’s action means the drug has now been fully vetted by the U.S. government and can remain on the market indefinitely.
Baca lebih lajut »
 Paxlovid, Pfizer's COVID-19 pill, gets full FDA approvalU.S. health regulators have given full approval to Pfizer’s COVID-19 pill Paxlovid.
Paxlovid, Pfizer's COVID-19 pill, gets full FDA approvalU.S. health regulators have given full approval to Pfizer’s COVID-19 pill Paxlovid.
Baca lebih lajut »
 Pfizer's Paxlovid still free, for now, after FDA grants full approval to COVID drugThe Biden administration will continue to manage the distribution of free courses of Pfizer's Paxlovid treatment for COVID-19 for at least another few months, the drugmaker said, even after the FDA granted Pfizer full approval Thursday to market the pills.
Pfizer's Paxlovid still free, for now, after FDA grants full approval to COVID drugThe Biden administration will continue to manage the distribution of free courses of Pfizer's Paxlovid treatment for COVID-19 for at least another few months, the drugmaker said, even after the FDA granted Pfizer full approval Thursday to market the pills.
Baca lebih lajut »
 COVID pill Paxlovid gets full FDA approval after more than a year of emergency useMore than 14,000 new COVID-19 cases were reported each week last month, although most U.S. cases are no longer reported to health authorities.
COVID pill Paxlovid gets full FDA approval after more than a year of emergency useMore than 14,000 new COVID-19 cases were reported each week last month, although most U.S. cases are no longer reported to health authorities.
Baca lebih lajut »
 Pfizer's COVID antiviral Paxlovid gains full US approvalPaxlovid initially received emergency use authorization from the FDA in late 2021, when there was a desperate need for effective COVID treatments. Paxlovid, taken for five days beginning shortly after onset of symptoms, was one of the few treatments launched by drugmakers during the pandemic to show a significant reduction in hospitalizations and deaths from COVID, although the benefit was mostly observed in unvaccinated and other higher-risk people. U.S. officials have said they plan to work through much of the Paxlovid inventory purchased from Pfizer, which is available for free at pharmacies around the country, before moving to a normal commercial market.
Pfizer's COVID antiviral Paxlovid gains full US approvalPaxlovid initially received emergency use authorization from the FDA in late 2021, when there was a desperate need for effective COVID treatments. Paxlovid, taken for five days beginning shortly after onset of symptoms, was one of the few treatments launched by drugmakers during the pandemic to show a significant reduction in hospitalizations and deaths from COVID, although the benefit was mostly observed in unvaccinated and other higher-risk people. U.S. officials have said they plan to work through much of the Paxlovid inventory purchased from Pfizer, which is available for free at pharmacies around the country, before moving to a normal commercial market.
Baca lebih lajut »
 Pfizer's COVID antiviral Paxlovid gains full US approvalThe U.S. Food and Drug Administration on Thursday granted full approval to Pfizer's oral antiviral COVID-19 treatment Paxlovid, clearing the way for the drugmaker to sell it at market rates once U.S. government supplies dwindle.
Pfizer's COVID antiviral Paxlovid gains full US approvalThe U.S. Food and Drug Administration on Thursday granted full approval to Pfizer's oral antiviral COVID-19 treatment Paxlovid, clearing the way for the drugmaker to sell it at market rates once U.S. government supplies dwindle.
Baca lebih lajut »
