CDC Director Dr. Rochelle Walensky’s final approval, which could come within hours, will be a milestone in the U.S. vaccination campaign.
A Centers for Disease Control and Prevention advisory committee on Saturday endorsed Pfizer-BioNTech and Moderna’s Covid-19 vaccines for young children, the last step before Director Dr. Rochelle Walensky can issue her final signoff.
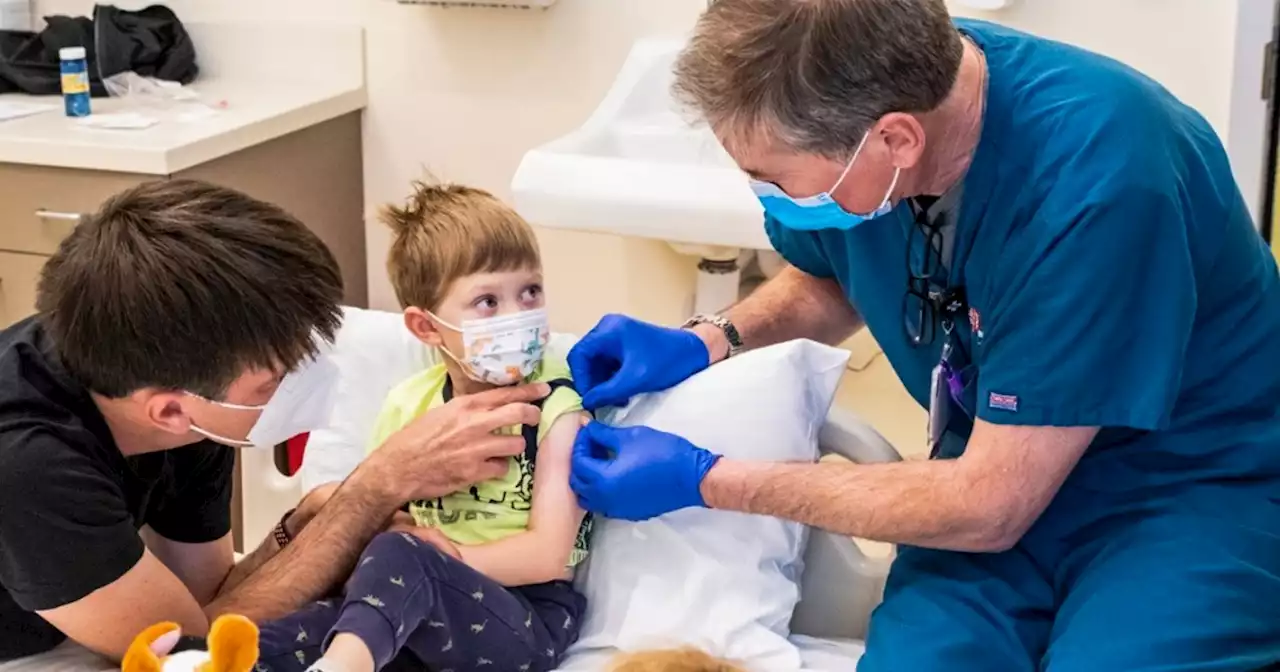
Three-year-old Andel was the first youngster to receive the Pfizer-BioNTech Covid-19 vaccine at Stanford Medicine. His parents, Otavio and Zina Good, enrolled him in a clinical trial for children aged 6 months through 4 years. Steve Fisch / Stanford Medicine Walensky is expected to endorse the recommendation shortly, a move that would allow vaccinations to finally begin.
Indonesia Berita Terbaru, Indonesia Berita utama
Similar News:Anda juga dapat membaca berita serupa dengan ini yang kami kumpulkan dari sumber berita lain.
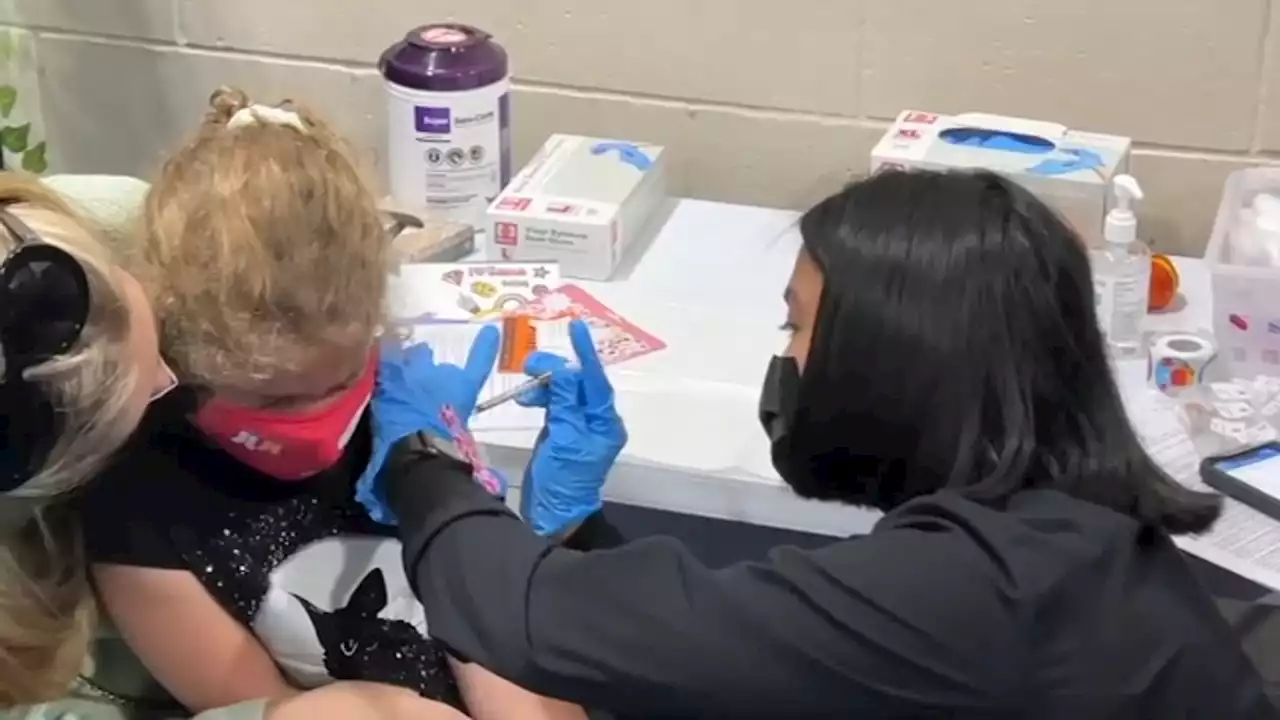 CDC panel meeting on COVID-19 vaccine for younger kidsThe next steps towards approving COVID vaccines for young children are underway and in the hands of the CDC.
CDC panel meeting on COVID-19 vaccine for younger kidsThe next steps towards approving COVID vaccines for young children are underway and in the hands of the CDC.
Baca lebih lajut »
 CDC panel meeting Saturday to recommend COVID vaccines for children under 5The CDC's vaccine advisers are expected to vote Saturday to allow children under 5 to receive COVID-19 vaccines.
CDC panel meeting Saturday to recommend COVID vaccines for children under 5The CDC's vaccine advisers are expected to vote Saturday to allow children under 5 to receive COVID-19 vaccines.
Baca lebih lajut »
 COVID-19 vaccine: CDC panel approves 1st shots for US kids under 5With the CDC's final sign-off, roughly 18 million children under 5 would be eligible for COVID-19 shots.
COVID-19 vaccine: CDC panel approves 1st shots for US kids under 5With the CDC's final sign-off, roughly 18 million children under 5 would be eligible for COVID-19 shots.
Baca lebih lajut »
 CDC panel recommends COVID-19 shots for children under 5The CDC's vaccine advisers have recommended COVID-19 vaccines for children under 5.
CDC panel recommends COVID-19 shots for children under 5The CDC's vaccine advisers have recommended COVID-19 vaccines for children under 5.
Baca lebih lajut »
 CDC advisers vote unanimously to recommend COVID vaccines for kids under 5 years oldShots can be administered to kids as young as 6 months old once the CDC director signs off on the panel's recommendations.
CDC advisers vote unanimously to recommend COVID vaccines for kids under 5 years oldShots can be administered to kids as young as 6 months old once the CDC director signs off on the panel's recommendations.
Baca lebih lajut »
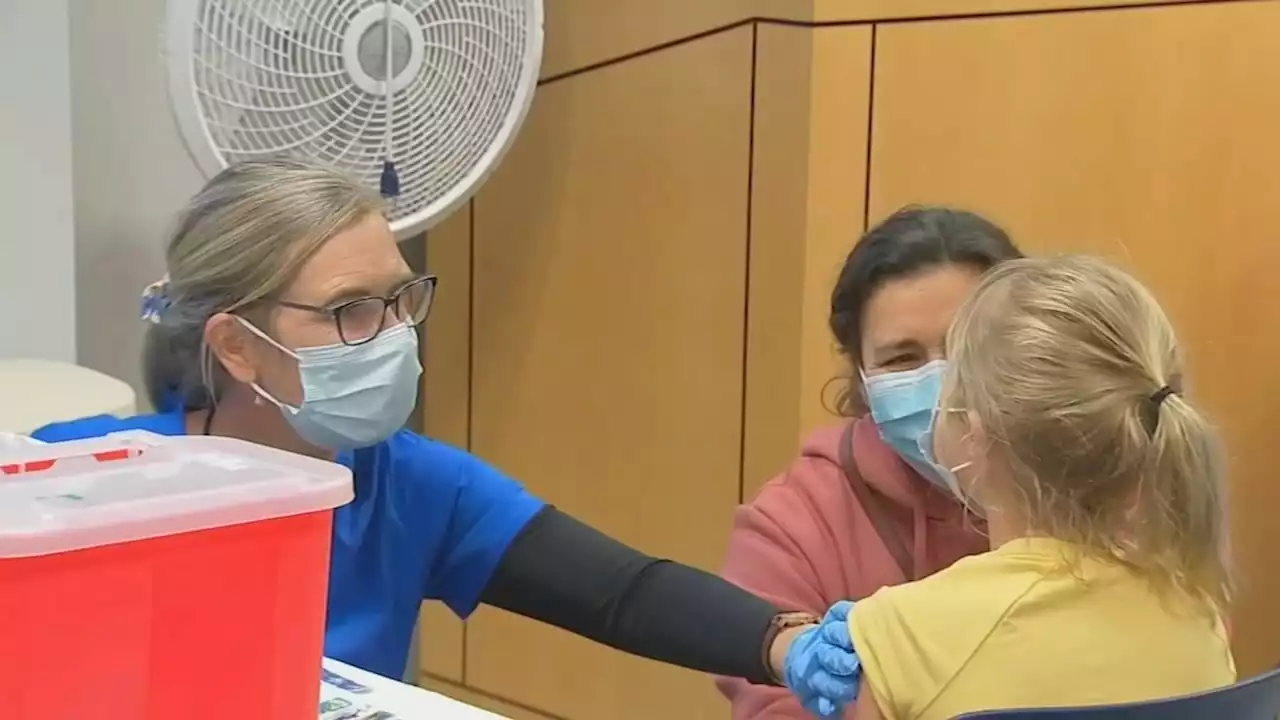 CDC advisers recommend Pfizer, Moderna COVID shots for kids under 5The CDC recommended COVID-19 vaccines for infants, toddlers and preschoolers on Saturday.
CDC advisers recommend Pfizer, Moderna COVID shots for kids under 5The CDC recommended COVID-19 vaccines for infants, toddlers and preschoolers on Saturday.
Baca lebih lajut »