Here's what you need to know about the recent clearance of COVID19 vaccines for kids under 5.
Late last week, the for both Moderna and Pfizer/BioNTech’s mRNA vaccines for the youngest age groups. The Moderna vaccine had its EUA amended to include individuals from 6 months to 17 years and the Pfizer/BioNTech, which was previously available to kids 5 and older, was cleared for kids who are 6 months to 4 years old.
“Many parents, caregivers and clinicians have been waiting for a vaccine for younger children and this action will help protect those down to 6 months of age. As we have seen with older age groups, we expect that the vaccines for younger children will provide protection from the most severe outcomes of COVID-19, such as hospitalization and death,” FDA Commissioner Robert M. Califf, M.D. said in a statement following the move.
Much like with teens and adults, both vaccines are considered equally safe and effective but there are slight differences in the dosages and timing. For the Moderna vaccine, it’s administered via two doses, each a month apart and a third booster for immunocompromised individuals in the age group a month after their second dose.
“As with all vaccines for any population, when authorizing COVID-19 vaccines intended for pediatric age groups, the FDA ensures that our evaluation and analysis of the data is rigorous and thorough,” Peter Marks, M.D., Ph.D., director of the FDA’s Center for Biologics Evaluation and Research said in a statement.
Indonesia Berita Terbaru, Indonesia Berita utama
Similar News:Anda juga dapat membaca berita serupa dengan ini yang kami kumpulkan dari sumber berita lain.
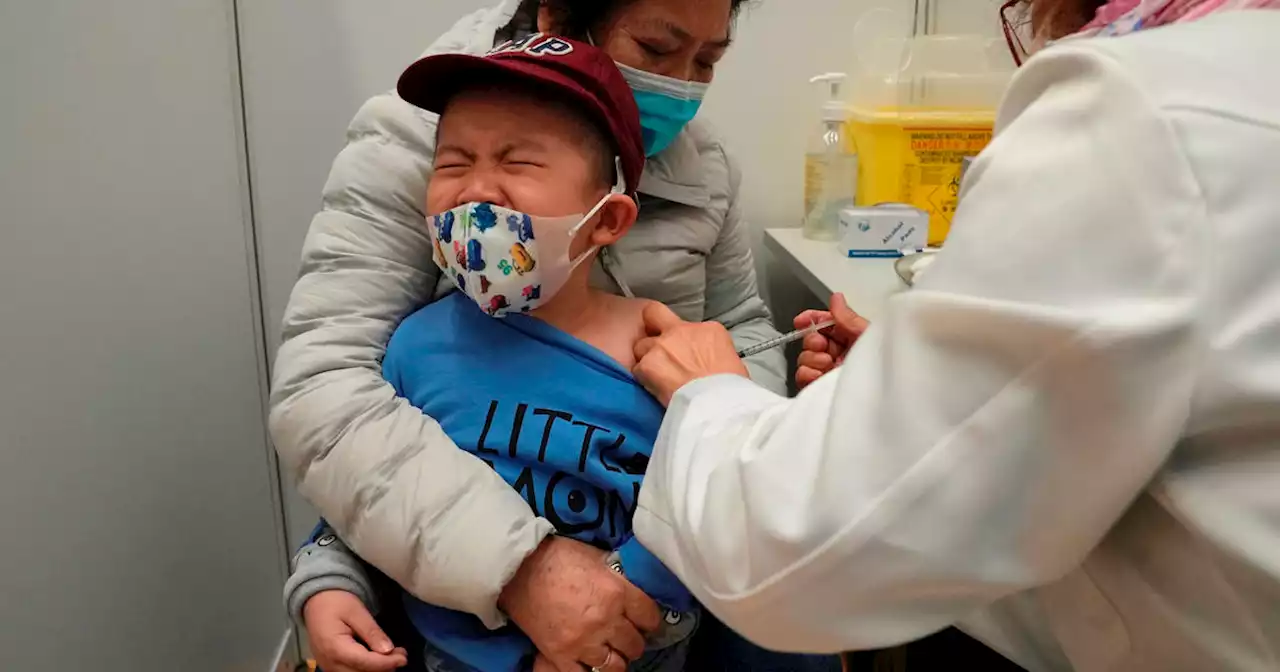 Bay Area kids under 5 to begin getting COVID vaccine following CDC, FDA approvalBay Area children under age 5 will have access to the COVID-19 vaccine for the first time this week following federal regulators' approval of the Pfizer-BioNTech and Moderna vaccines.
Bay Area kids under 5 to begin getting COVID vaccine following CDC, FDA approvalBay Area children under age 5 will have access to the COVID-19 vaccine for the first time this week following federal regulators' approval of the Pfizer-BioNTech and Moderna vaccines.
Baca lebih lajut »
 CDC director approves COVID-19 vaccines for children under 5Centers for Disease Control and Prevention Director Rochelle Walensky on Saturday approved Moderna and Pfizer-BioNTech’s COVID-19 vaccines for use in American children as young as 6 months.
CDC director approves COVID-19 vaccines for children under 5Centers for Disease Control and Prevention Director Rochelle Walensky on Saturday approved Moderna and Pfizer-BioNTech’s COVID-19 vaccines for use in American children as young as 6 months.
Baca lebih lajut »
 CDC clears the way for vaccinations for children 6 months to 5 years oldAn independent panel of advisers to the U.S. Centers for Disease Control and Prevention voted on Saturday to recommend vaccinating all children in the age group with one of two separate COVID-19 vaccines.
CDC clears the way for vaccinations for children 6 months to 5 years oldAn independent panel of advisers to the U.S. Centers for Disease Control and Prevention voted on Saturday to recommend vaccinating all children in the age group with one of two separate COVID-19 vaccines.
Baca lebih lajut »
 Your Life, Your Lifestyle: How Men Can Lower Their Chance of Getting Cancer | Blogs | CDCThis MensHealthMonth, help your male patients lower their risk of cancer by encouraging healthy lifestyle choices and helping them stay up to date on cancer screening tests. Find more tips to share with patients from CDCgov:
Your Life, Your Lifestyle: How Men Can Lower Their Chance of Getting Cancer | Blogs | CDCThis MensHealthMonth, help your male patients lower their risk of cancer by encouraging healthy lifestyle choices and helping them stay up to date on cancer screening tests. Find more tips to share with patients from CDCgov:
Baca lebih lajut »
 The Number of Children Poisoned By This Product Grew By 500% In the Last Decade, CDC Says — Eat This Not ThatMore people are using wellness supplements than ever before. But just because they are sold over the counter doesn't mean they are safe.
The Number of Children Poisoned By This Product Grew By 500% In the Last Decade, CDC Says — Eat This Not ThatMore people are using wellness supplements than ever before. But just because they are sold over the counter doesn't mean they are safe.
Baca lebih lajut »
