JUST IN: The advisers to the CDC unanimously decided that coronavirus vaccines should be opened to children as young as 6 months. The final signoff is expected later in the day from CDC Director Dr. Rochelle Walensky.
This May 2022 photo provided by Pfizer shows production of the Pfizer's COVID-19 vaccine for children under 5 in Puurs, Belgium. U.S. regulators on Friday, June 17, authorized the first COVID-19 shots for infants and preschoolers, paving the way for vaccinations to begin next week. – U.S. health advisers on Saturday recommended COVID-19 vaccines for infants, toddlers and preschoolers — the last group without the shots.
The final signoff was expected later in the day from CDC Director Dr. Rochelle Walensky. While the Food and Drug Administration OKs vaccines, it's the CDC that decides who should get them.for distribution to doctors, hospitals and community health clinics around the country. Roughly 18 million kids will be eligible.
Moderna’s is two shots, each a quarter of its adult dose, given about four weeks apart for kids 6 months through 5. The FDA also approved a third dose, at least a month after the second shot, for kids with immune conditions that make them more vulnerable to serious illness.In studies, vaccinated youngsters developed levels of virus-fighting antibodies as strong as young adults, suggesting that the kid-size doses protect against coronavirus infections.
Hospitalizations surged during the omicron wave. Since the start of the pandemic, about 480 children under age 5 are counted among the nation’s more than 1 million COVID-19 deaths, federal data show. U.S. officials expect most shots to take place at pediatricians’ offices. Many parents may be more comfortable getting the vaccine for their kids at their regular doctor, White House COVID-19 coordinator Dr. Ashish Jha said. He predicted the pace of vaccination to be far slower than it was for older populations.
Indonesia Berita Terbaru, Indonesia Berita utama
Similar News:Anda juga dapat membaca berita serupa dengan ini yang kami kumpulkan dari sumber berita lain.
 COVID-19 vaccine: FDA authorizes 1st shots for kids under 5; CDC review nextBREAKING: U.S. regulators on Friday authorized the first COVID-19 shots for infants and preschoolers, paving the way for vaccinations to begin next week.
COVID-19 vaccine: FDA authorizes 1st shots for kids under 5; CDC review nextBREAKING: U.S. regulators on Friday authorized the first COVID-19 shots for infants and preschoolers, paving the way for vaccinations to begin next week.
Baca lebih lajut »
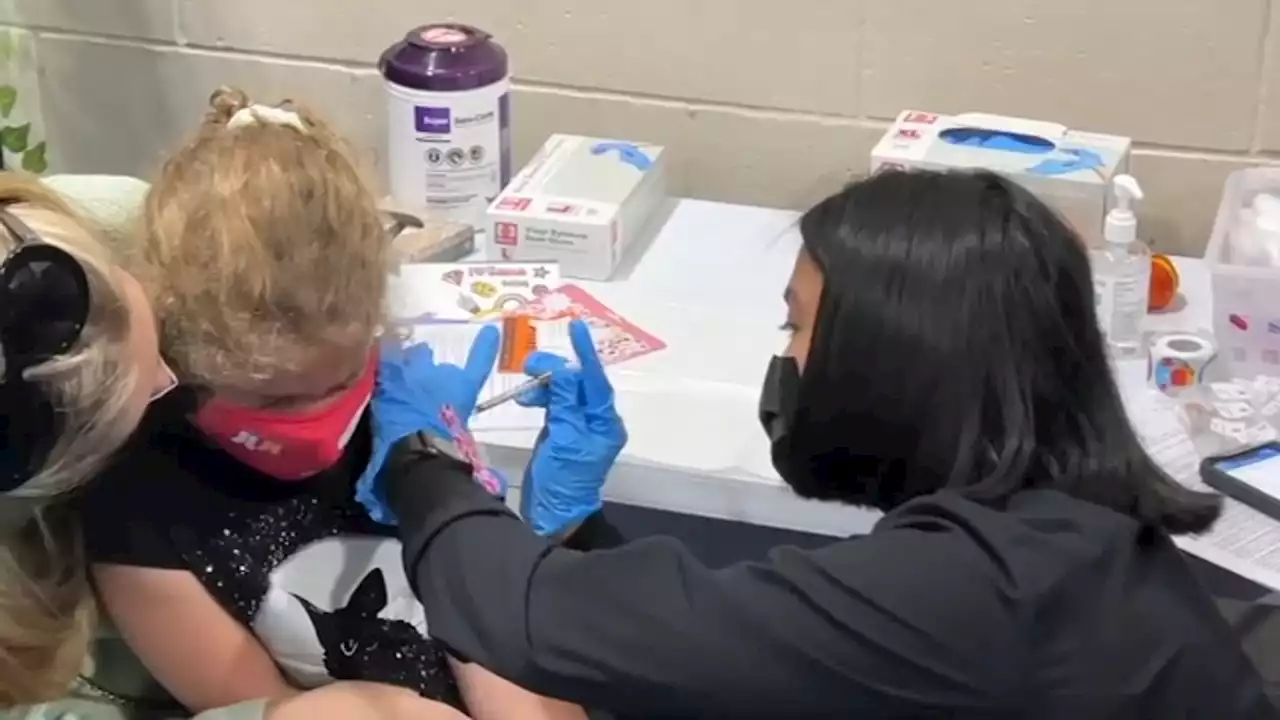 CDC panel meeting on COVID-19 vaccine for younger kidsThe next steps towards approving COVID vaccines for young children are underway and in the hands of the CDC.
CDC panel meeting on COVID-19 vaccine for younger kidsThe next steps towards approving COVID vaccines for young children are underway and in the hands of the CDC.
Baca lebih lajut »
 FDA authorizes 1st COVID-19 shots for kids under 5; CDC review is nextU.S. regulators on Friday authorized the first COVID-19 shots for infants and preschoolers, paving the way for vaccinations to begin next week.
FDA authorizes 1st COVID-19 shots for kids under 5; CDC review is nextU.S. regulators on Friday authorized the first COVID-19 shots for infants and preschoolers, paving the way for vaccinations to begin next week.
Baca lebih lajut »
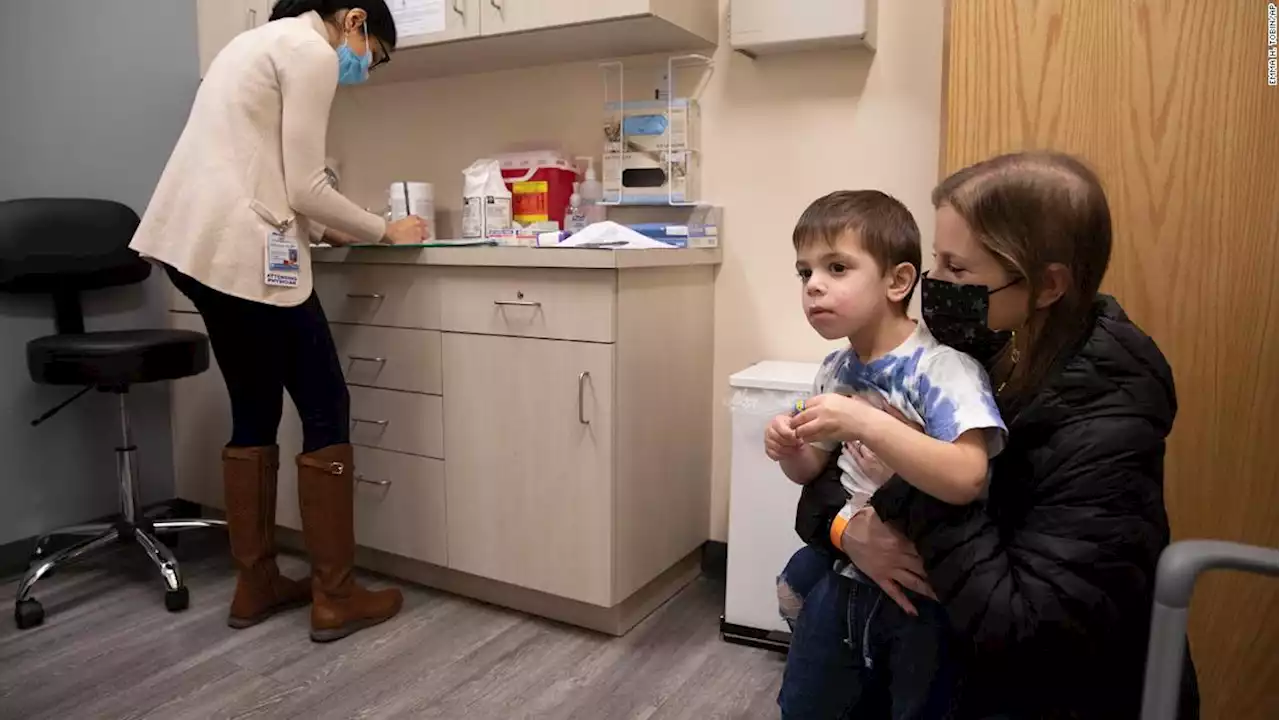 CDC advisers vote to recommend Covid-19 vaccines for children as young as 6 monthsVaccine advisers to the US Centers for Disease Control and Prevention voted unanimously on Saturday in support of recommending the Moderna and Pfizer/BioNTech Covid-19 vaccines to children as young as 6 months.
CDC advisers vote to recommend Covid-19 vaccines for children as young as 6 monthsVaccine advisers to the US Centers for Disease Control and Prevention voted unanimously on Saturday in support of recommending the Moderna and Pfizer/BioNTech Covid-19 vaccines to children as young as 6 months.
Baca lebih lajut »
 COVID-19 vaccine: CDC panel approves 1st shots for US kids under 5With the CDC's final sign-off, roughly 18 million children under 5 would be eligible for COVID-19 shots.
COVID-19 vaccine: CDC panel approves 1st shots for US kids under 5With the CDC's final sign-off, roughly 18 million children under 5 would be eligible for COVID-19 shots.
Baca lebih lajut »
 CDC panel recommends COVID-19 shots for children under 5The CDC's vaccine advisers have recommended COVID-19 vaccines for children under 5.
CDC panel recommends COVID-19 shots for children under 5The CDC's vaccine advisers have recommended COVID-19 vaccines for children under 5.
Baca lebih lajut »
